��Ŀ����
��100 mL FeI2��Һ����ͨ��Cl2������������I2��Fe3����IO3-������Fe3����I2�����ʵ�����n(Cl2)�ı仯��ͼ��ʾ����ش��������⣺
��1����ͼ��֪��I����Fe2����I2�������ӵĻ�ԭ����ǿ������˳��Ϊ________��________��________��
��2����n(Cl2)��0.12 molʱ����Һ�е�������ҪΪ________________________________��
�ӿ�ʼͨ��Cl2��n(Cl2)��0.12 molʱ���ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________��
��3������Һ��n(Cl��)��n(IO3-)��8��1ʱ��ͨ���Cl2�ڱ�״���µ����Ϊ________��
��1��I����Fe2����I2
��2��Fe2����Fe3����Cl����5FeI2��6Cl2=5I2��2FeCl3��3FeCl2
��3��8.96 L
����

��ϰ��ϵ�д�
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�����Ŀ
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�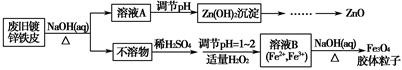
��֪��Zn���仯�����������Al���仯������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
��3������ҺB�Ƶ�Fe3O4�������ӵĹ����У������ͨ��N2����ԭ����_________________________________________________________��
��4�����ظ���ط���һ��������ԭ�ζ������ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.010 00 mol��L��1��K2Cr2O7����Һ250 mL��Ӧȷ��ȡ________g K2Cr2O7������4λ��Ч���֣���֪MK2Cr2O7��294.0 g��mol��1�������Ƹñ���Һʱ�����������в���Ҫ�õ�����________���ñ�ű�ʾ����
�ٵ�����ƽ�����ձ�������Ͳ���ܲ�������������ƿ����ͷ�ιܣ�����Һ��
��5���ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ⶨ�����________���ƫ��ƫС�����䡱����
 FeCl3
FeCl3 TiO2��H2O
TiO2��H2O
 ���뼾���Ĵ���
���뼾���Ĵ��� �������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
�������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
 3Cu2++2R+yH2O��
3Cu2++2R+yH2O��