��Ŀ����
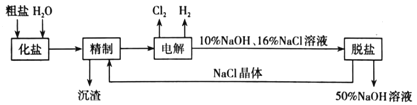
����Ŀ������������һ�ְ�ɫ���壬������ˮ���������Ҵ�����ѧ����������������ơ�ijѧϰС������ڼ��Ի���������CaCl2��H2O2��Ӧ��ȡCaO2��8H2O��װ����ͼ��ʾ��
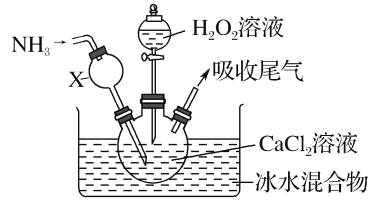
�ش��������⣺
��1��С��ͬѧ��������֪����ʵ������������Ϊ20%��H2O2��Һ��Ϊ���ˡ�����H2O2��Һ����������Ϊ30%����С��ͬѧ������H2O2��Һ����Լ20%��H2O2��Һ�Ĺ����У�ʹ�õIJ�������������������ͷ�ι��⣬����___��
��2������X����Ҫ���ó������⣬�����е�������___��
��3���ڱ�ˮԡ�н��е�ԭ����___��
��4��ʵ��ʱ����������ƿ������CaO2��8H2O���壬�ܷ�Ӧ�����ӷ���ʽΪ___��
��5����Ӧ���������ˡ�ϴ�ӡ����º�ɻ��CaO2��8H2O�������Լ��У�ϴ��CaO2��8H2O�����ѡ����____��
A����ˮ�Ҵ� B��Ũ���� C��Na2SO3��Һ D��CaCl2��Һ
��6����CaCl2ԭ���к���Fe3+���ʣ�Fe3+���ֽ�H2O2����ʹH2O2�����������Խ��͡���Ӧ�Ļ���Ϊ��
��Fe3+ +H2O2=Fe2++H++HOO��
��H2O2+X=Y +Z+W������ƽ��
��Fe2++��OH=Fe3++OH-
��H+ +OH-=H2O
�������������Ƶ�������еĻ�ѧ����ʽΪ___��
��7���������ƿ����ڳ�;�������磬�������˹������ƾ���____�����ʡ�
A.��ˮ������Ӧ���� B.���������������CO2����
C.����ˮ��������ǿ D.����ǿ�����ԣ���ɱ������
��8��������CaO2��8H2O������ȵ�150~160�棬��ȫ��ˮ��õ�����������Ʒ��
��С��ⶨ����������Ʒ��CaO2�Ĵ��ȵķ����ǣ�ȷ��ȡ0.4000g����������Ʒ��400�����ϼ�������ȫ�ֽ��CaO��O2(�����ʲ���������)���õ�33.60mL(�ѻ���Ϊ��״��)���塣
�����ù���������Ʒ��CaO2�Ĵ���Ϊ_____��
���𰸡��ձ�����Ͳ ��ֹ������ƿ����Һ�������� ��ֹ�¶ȹ���H2O2�ֽ⡢�����ھ������� Ca2++H2O2+2NH3+8H2O=CaO2��8H2O��+2NH4+ A HOO��+ H2O2=H2O + O2 +��OH ABD 54.00%
��������
����һ��������������Һ��Ҫ��������ֻ��Ҫ�ӳ���֪ʶ���
ͨ�백���Ӱ������ܽ���˼����
˫��ˮ�ڱ�ˮԡ�У���˫��ˮ�IJ��ȶ������⣬
����ԭ�ϺͲ�����д���ɰ�ˮ�������Ƶ����ӷ���ʽ��
�������ƾ�����������ʵó�ϴ�Ӱ�ˮ�������Ƶ��Լ���
�������ǰ���ϵ��˫��ˮ�ֽ�����ˮ��������֪ʶ�ó��м���T��Ӧ����ʽ��
���ù�ϵʽ���㴿�ȡ�
������Լ20%��H2O2��Һ�Ĺ����У�ʹ�õIJ�������������������ͷ�ιܡ��ձ�����Ͳ��
�ʴ�Ϊ���ձ�����Ͳ��
������X����Ҫ���ó������⣬��Ϊ������������ˮ����˻����з��������ã�
�ʴ�Ϊ����ֹ������ƿ����Һ����������
��˫��ˮ���ȷֽ⣬����ڱ�ˮԡ�н��е�ԭ���Ƿ�ֹ�¶ȹ���H2O2�ֽ⡢�����ھ���������
�ʴ�Ϊ����ֹ�¶ȹ���H2O2�ֽ⡢�����ھ�����������
��ʵ��ʱ����������ƿ������CaO2��8H2O���壬�ܷ�Ӧ�����ӷ���ʽΪCa2++H2O2+2NH3+ 8H2O =CaO2��8H2O��+2NH4+��
�ʴ�Ϊ��Ca2++H2O2+2NH3+8H2O=CaO2��8H2O��+2NH4+��
�ɹ���������һ�ְ�ɫ���壬������ˮ���������Ҵ������ϴ��CaO2��8H2O�����ʵ��Ϊ��ˮ�Ҵ���
�ʴ�Ϊ��A��
����CaCl2ԭ���к���Fe3+���ʣ�Fe3+���ֽ�H2O2��Ϊ������ˮ������ǰ����ϵ��˵�����в�����������ˮ������OH���仯ѧ����ʽΪ��HOO��+ H2O2=H2O + O2 +��OH��
�ʴ�Ϊ��HOO��+ H2O2=H2O + O2 +��OH��
�˹������ƿ����ڳ�;�������磬������Ҫ������˵���������ƾ�����ˮ������Ӧ��������������������Ķ�����̼�����ɱ�����ã�
�ʴ�Ϊ��ABD��
�̽�����CaO2��8H2O������ȵ�150~160������ȫ��ˮ��õ�����������Ʒ��
��С��ⶨ����������Ʒ��CaO2�Ĵ��ȵķ����ǣ�ȷ��ȡ0.4000g����������Ʒ��400�����ϼ�������ȫ�ֽ��CaO��O2(�����ʲ���������)���õ�33.60mL�����ʵ���Ϊ1.5��10-3 mol����
2CaO2 = 2CaO + O2
���ݹ�ϵ�ó�n(CaO2) = 3��10-3 mol��
![]() ��
��
�ʴ�Ϊ54.00%��

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�