��Ŀ����
����Ŀ��Һ����Һ��ȼ�ϵ������������DZͧ����ʾ��ͼ��ͼ��ʾ��
(1)�õ�ص��ܷ�ӦʽΪ_____________���缫1�����ĵ缫��ӦΪ__________��
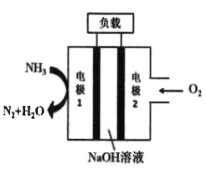
(2)����˵����ȷ����______________��
A. �缫2��������������ԭ��Ӧ
B. ��ع���ʱ��Na����缫1�ƶ�
C. �����ɵ缫2�����·����缫1
D. ��װ�ý���ѧ��ת��Ϊ������ת��Ϊ��е��
(3)����·��ͨ���ĵ�����Ϊ0.4molʱ�������������ı�״�������������Ϊ________L��
���𰸡�4NH3 + 3O2 = 2N2 + 6H2O 2NH3 - 6e- + 6OH- = N2��+ 6H2O ACD 2.24
��������
ȼ�ϵ�ص��ܷ�Ӧ��ȼ��ȼ�յĻ�ѧ����ʽ������ͼʾ��������������Ӧ���ɵ�����ˮ���ݴ���д��Ӧ�ķ���ʽ��ȼ�ϵ����ͨ�������ĵ缫Ϊ�������缫2���������缫1�Ǹ�������ȼ�ϵ�صĸ����Ϸ���ȼ��ʧ���ӵ�������Ӧ���缫����ʽΪ2NH3+6OH--6e-=N2��+6H2O�������������������õ��ӵĻ�ԭ��Ӧ���缫��ӦʽΪO2+4e-+2H2O=4OH-���ݴ˷������
(1)ȼ�ϵ�ص��ܷ�Ӧ��ȼ��ȼ�յĻ�ѧ����ʽ����4NH3+3O2=2N2+6H2O����ȼ�ϵ�صĸ����Ϸ���ȼ�ϰ���ʧ���ӵ�������Ӧ������Ի����µ缫1�ĵ缫��ӦʽΪ��2NH3+6OH--6e-=N2��+6H2O���ʴ�Ϊ��4NH3+3O2=2N2+6H2O��2NH3+6OH--6e-=N2��+6H2O��
(2) A.ȼ�ϵ����ͨ�������ĵ缫Ϊ��������˵缫2Ϊ�����������Ϸ����õ��ӵĻ�ԭ��Ӧ����A��ȷ��B���缫1Ϊ������ԭ��ع���ʱ���������������ƶ�����Na+��缫2�ƶ�����B����C�������������������������ɵ缫2�����·����缫1����C��ȷ��D. ȼ�ϵ������ԭ��أ�ԭ����ǽ���ѧ��ת��Ϊ���ܵ�װ�ã�������ת��Ϊ��е������DZͧ����D��ȷ���ʴ�Ϊ��ACD��
(3) 4NH3 + 3O2 = 2N2 + 6H2O��ת�Ƶĵ�����Ϊ12������·��ͨ���ĵ�����Ϊ0.4molʱ������������������![]() =0.1mol����״���µ����Ϊ22.4L/mol��0.1mol=2.24L���ʴ�Ϊ��2.24��
=0.1mol����״���µ����Ϊ22.4L/mol��0.1mol=2.24L���ʴ�Ϊ��2.24��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ѧ����С���������ͼ��ʾ��װ����ȡ��������(ͼ�мг������ͼ���װ������ȥ)����ش�����������
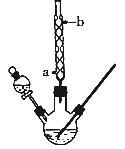
��1�����������ܵ�������____________������ˮ��____________(����a������b��)�����롣
��2����֪����������
�Ҵ� | ���� | �������� | 98%Ũ���� | |
�ܵ�/�� | 117.3 | 16.6 | -83.6 | |
�е�/�� | 78.5 | 117.9 | 77.5 | 338.0 |
��֪�¶ȸ���140��ʱ��������Ӧ��2CH3CH2OH��CH3CH2OCH2CH3+H2O
���ø���Ӧ����____________��Ӧ(����ĸ)��
a���ӳɷ�Ӧ b��ȡ����Ӧ c��������Ӧ d����ȥ��Ӧ
�����ǵ���Ӧ���ʵȶ������أ�������װ���Ʊ���������ʱ����Ӧ������¶ȷ�Χ��____________(����ĸ)��
a��T<77.5�� b��T>150�� c��115��<T<130��
��3����Ӧ���������ӷ�Ӧ������з��������������Ӧʹ�õķ��뷽����____________(������������ƣ���ͬ)�����õ�����Ҫ����Ϊ____________�����д˲������������л����е���Ҫ����������ˮ���ڲ�����ʹ�ø�����������£���ȥˮ����____________�ķ�����������������ͬ�����ʵ�ͬ���칹���У���һ��ֱ��������ǿ��������������������ͭ��Һ��ϼ���һ��ʱ�����������ש��ɫ��������д���Ļ�ѧ����ʽ________________________��
��4����ʵ��������������Ϊ6.0g���Ҵ�����Ϊ5.0g���õ������IJ�Ʒ����Ϊ4.4g�������������IJ�����____________��
��5���Ҷ�����Ҷ���Ҳ�ܷ���������Ӧ��д�����ɻ����Ļ�ѧ��Ӧ����ʽ______________________��