��Ŀ����
�л�������A��̼���⡢������Ԫ����ɣ���ȡ3g A��4.48L��������״�������ܱ�������ȼ�գ�ȼ�պ����ɶ�����̼��һ����̼��ˮ���������跴Ӧ��û��ʣ�ࣩ������Ӧ���ɵ���������ͨ��Ũ����ͼ�ʯ�ң�Ũ��������3.6g����ʯ������4.4g���ش��������⣺
��1��3g A��������ԭ�ӡ�̼ԭ�ӵ����ʵ����ֱ��� �� ��
��2��ͨ������ȷ�����л���ķ���ʽΪ ��
��3����A�����������ܷ���������Ӧ�����л�������A�Ľṹ��ʽ��
��1��3g A��������ԭ�ӡ�̼ԭ�ӵ����ʵ����ֱ���
��2��ͨ������ȷ�����л���ķ���ʽΪ
��3����A�����������ܷ���������Ӧ�����л�������A�Ľṹ��ʽ��
���㣺�й��л������ʽȷ���ļ���
ר�⣺�������������ȼ�չ���
��������1��Ũ��������3.6gΪˮ����������ʯ������4.4gΪ������̼������������n=
���������̼��ˮ�����ʵ���������n=
�������������ʵ��������������غ����CO������������n=
����CO�����ʵ������ٸ���ԭ���غ���㣻
��2��������ԭ���غ����3gA����ԭ�ӵ��������ٸ��ݣ�1���еļ���ȷ�������ʵ����ʽ���ݴ˽��
��3����A�����������ܷ���������Ӧ�������������к���-CHO��˵��A���ǻ����ӵ�̼ԭ������2��Hԭ�ӣ�����л������ʽȷ����ṹ��ʽ��
| m |
| M |
| V |
| Vm |
| m |
| M |
��2��������ԭ���غ����3gA����ԭ�ӵ��������ٸ��ݣ�1���еļ���ȷ�������ʵ����ʽ���ݴ˽��
��3����A�����������ܷ���������Ӧ�������������к���-CHO��˵��A���ǻ����ӵ�̼ԭ������2��Hԭ�ӣ�����л������ʽȷ����ṹ��ʽ��
���
�⣺��1��Ũ��������3.6gΪˮ��������n��H2O��=
=0.2mol��
��ʯ������4.4gΪ������̼��������n��CO2��=
=0.1mol��
4.48L���������ʵ���=
=0.2mol������������=0.2mol��32g/mol=6.4g
��CO������=3g+6.4g-3.6g-4.4g=1.4g���� n��CO��=
=0.05mol��
��3g A��n��H��=2n��H2O��=0.4mol��n��C��=n��CO2��+n��CO��=0.1mol+0.05mol=0.15mol��
�ʴ�Ϊ��0.4mol��0.15mol
��2��3g A��n��H��=0.4mol��n��C��=0.15mol��
3g A��n��O��=2n��CO2��+n��CO��+n��H2O��-2n��O2��=2��0.1 mol+0.05 mol+0.2 mol-2��0.2 mol=0.05mol��
���ԣ�n��C����n��H����n��O��=3��8��1��
A�����ʽΪC3H8O����Hԭ����̼ԭ����Ŀ��֪��A�ķ���ʽΪC3H8O��
�ʴ�Ϊ��C3H8O��
��3����A�����������ܷ���������Ӧ�������������к���-CHO��˵��A���ǻ����ӵ�̼ԭ������2��Hԭ�ӣ���A�Ľṹ��ʽΪCH3CH2CH2OH��
���л�������A�Ľṹ��ʽΪCH3CH2CH2OH��
| 3.6g |
| 18g/mol |
��ʯ������4.4gΪ������̼��������n��CO2��=
| 4.4g |
| 44g/mol |
4.48L���������ʵ���=
| 4.48L |
| 22.4L/mol |
��CO������=3g+6.4g-3.6g-4.4g=1.4g���� n��CO��=
| 1.4g |
| 28g/mol |
��3g A��n��H��=2n��H2O��=0.4mol��n��C��=n��CO2��+n��CO��=0.1mol+0.05mol=0.15mol��
�ʴ�Ϊ��0.4mol��0.15mol
��2��3g A��n��H��=0.4mol��n��C��=0.15mol��
3g A��n��O��=2n��CO2��+n��CO��+n��H2O��-2n��O2��=2��0.1 mol+0.05 mol+0.2 mol-2��0.2 mol=0.05mol��
���ԣ�n��C����n��H����n��O��=3��8��1��
A�����ʽΪC3H8O����Hԭ����̼ԭ����Ŀ��֪��A�ķ���ʽΪC3H8O��
�ʴ�Ϊ��C3H8O��
��3����A�����������ܷ���������Ӧ�������������к���-CHO��˵��A���ǻ����ӵ�̼ԭ������2��Hԭ�ӣ���A�Ľṹ��ʽΪCH3CH2CH2OH��
���л�������A�Ľṹ��ʽΪCH3CH2CH2OH��
���������⿼���л������ʽȷ������������ͬ���칹����д�ȣ���Ŀ�ѶȲ���ע��������غ�ĽǶȼ���һ����̼���������ٸ���ԭ���غ���㣮

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
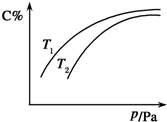 ���ܱ������У����ڿ��淴ӦA������+3B�������T2C��g����H��0��ƽ��ʱC������������¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������
���ܱ������У����ڿ��淴ӦA������+3B�������T2C��g����H��0��ƽ��ʱC������������¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������| A����n��A����n��B����n��c��=1��3��2ʱ����Ӧ�ﵽƽ��״̬ |
| B��A��B�ۼ�״̬����ȷ�� |
| C��ѹǿ����ʱ����������ƽ����Է����������� |
| D�������������䣬�����¶ȣ���Ӧ��ƽ�ⳣ������ |
��Ȼ����ʯ�͡�ú���ڵ����ϵ��̲��������ģ�������˵����ȷ���ǣ�������
�ٿ����õ��ˮ�ķ����Ƶ���������Դ
�ڿɿ�����ľ��Ұ������Դ
��Ӧ����̫���ܡ����ܵ�����Դ������Ӧ�÷��ܡ�ˮ�ܵȿ�������Դ��
�ٿ����õ��ˮ�ķ����Ƶ���������Դ
�ڿɿ�����ľ��Ұ������Դ
��Ӧ����̫���ܡ����ܵ�����Դ������Ӧ�÷��ܡ�ˮ�ܵȿ�������Դ��
| A���� | B���� | C���ڢ� | D���� |
���з�Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A��FeS��������ϡHNO3��FeS+2H+=Fe2++H2S�� |
| B��AlCl3������ˮ��Ӧ��Al3++3OH-=Al��OH��3�� |
| C����AgCl����Һ�еμ�������Һ����ɫ������ɺ�ɫ��2AgCl��s��+S2-��aq��=Ag2S��s��+2Cl-��aq�� |
| D�������Ũ�ȵ�NaHSO4��Ba��0H��2��Һ��ϣ�2H++SO42-+Ba2++2OH-=BaSO4��+2H2O |
 ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ������ʣ���ʵ��װ����ͼ�����Ⱦ۱�ϩ�����ϵõ��IJ������±���
ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ������ʣ���ʵ��װ����ͼ�����Ⱦ۱�ϩ�����ϵõ��IJ������±���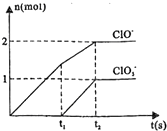 ��֪NaOH��Cl2��Ӧ�������������¶��йأ������ķ�Ӧ��Ϊ���ȷ�Ӧ������V L 4mol/L��NaOH��ͨ��һ�������������������к���Cl-��ClO-��ClO3-���ֺ���Ԫ�ص����ӣ�����C1O-��ClO3-�����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
��֪NaOH��Cl2��Ӧ�������������¶��йأ������ķ�Ӧ��Ϊ���ȷ�Ӧ������V L 4mol/L��NaOH��ͨ��һ�������������������к���Cl-��ClO-��ClO3-���ֺ���Ԫ�ص����ӣ�����C1O-��ClO3-�����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��