��Ŀ����
��һ��ɫ����Һ����֪���п��ܺ���Fe3+��Mg2+��Cu2+��Al3+��NH4+������һ�ֵ���ɫ��ĩ�����ȣ�������������������ʵ����뵭��ɫ��ĩ�����ʵ�����ϵ��ͼ��ʾ����ش�
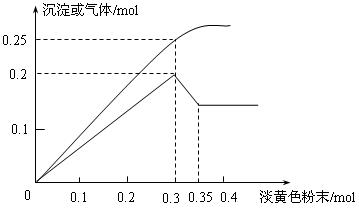
��1������ɫ��ĩΪ_____________�������ƣ�����Һ�п϶�û��____________���ӡ�
��2����Һ�д��ڵĸ����������ӵ����ʵ����ֱ���_________________________��
��3��������0.3mol����ɫ��ĩʱ����������ɷ��� �� ���ʵ���֮���� ��
��4���ٵ���ɫ��ĩ��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________________________��
�ڳ������ּ���ʱ�����ӷ���ʽΪ__________________________________��

��1������ɫ��ĩΪ_____________�������ƣ�����Һ�п϶�û��____________���ӡ�
��2����Һ�д��ڵĸ����������ӵ����ʵ����ֱ���_________________________��
��3��������0.3mol����ɫ��ĩʱ����������ɷ��� �� ���ʵ���֮���� ��
��4���ٵ���ɫ��ĩ��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________________________��
�ڳ������ּ���ʱ�����ӷ���ʽΪ__________________________________��
��1����1�֣��������� ��2�֣� Fe3����Cu2��
��2����2�֡�3=6�֣�Mg2���U0.1mol��NH4���U0.1mol��Al3���U0.1mol
��3����2�֡�2=4�֣�NH3, O2 ��2��3
(4)��2�֡�2=4�֣�
��2Na2O2+2H2O=4NaOH+O2�� ��Al(OH)3��OH��= AlO2����2H2O��
��2����2�֡�3=6�֣�Mg2���U0.1mol��NH4���U0.1mol��Al3���U0.1mol
��3����2�֡�2=4�֣�NH3, O2 ��2��3
(4)��2�֡�2=4�֣�
��2Na2O2+2H2O=4NaOH+O2�� ��Al(OH)3��OH��= AlO2����2H2O��
��

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
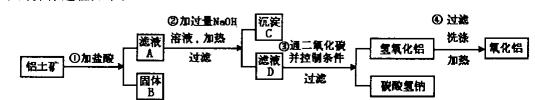
 ���
��� �ǡ�����������ԭ��Ӧ��д��̼�����Ƶ�һ����; ��
�ǡ�����������ԭ��Ӧ��д��̼�����Ƶ�һ����; ��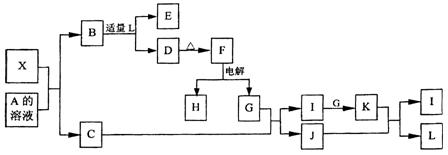
 4X��SiC��3C��
4X��SiC��3C��

 2MgO+Si(�����к���һ�����Ķ�������)��ͬʱ�и���Ӧ������2Mg+Si
2MgO+Si(�����к���һ�����Ķ�������)��ͬʱ�и���Ӧ������2Mg+Si Mg2Si��Mg2Si������Ѹ�ٷ�Ӧ����SiH4(����)��SiH4�ڳ�������һ�ֲ��ȶ����ֽ�����塣
Mg2Si��Mg2Si������Ѹ�ٷ�Ӧ����SiH4(����)��SiH4�ڳ�������һ�ֲ��ȶ����ֽ�����塣
 ����ȷ���� �� ��
����ȷ���� �� ��