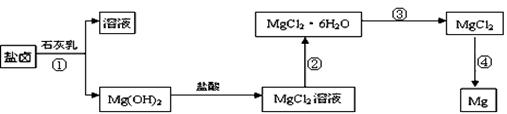
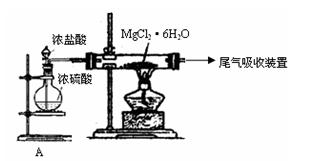
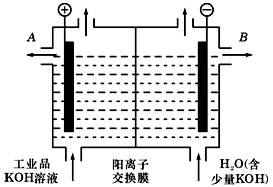
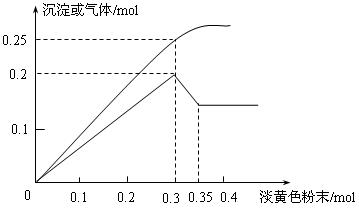
��Ŀ����
A��B��C��D�����ֳ������ʣ����ӦԪ�ص�ԭ������������������B��D���ڳ��������������Ϊ���������J��һ�ֺ�ɫ���壬I��Ũ��Һ���л�ԭ�ԣ���A��I����������֮�������µ�ת����ϵ��

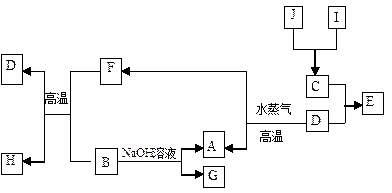
 (1)д���������ʵ�����A ��G ��
(1)д���������ʵ�����A ��G ��
 (2)д�����з�Ӧ�Ļ�ѧ����ʽ��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
 ��B+F��D+H�� ��
��B+F��D+H�� ��
 ��D��A+F�� ��
��D��A+F�� ��
 ��B��A+G�� ��
��B��A+G�� ��
 (3)������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��
(3)������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��  ��
��

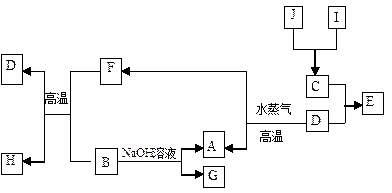
 (1)д���������ʵ�����A ��G ��
(1)д���������ʵ�����A ��G �� (2)д�����з�Ӧ�Ļ�ѧ����ʽ��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ�� ��B+F��D+H�� ��
��B+F��D+H�� �� ��D��A+F�� ��
��D��A+F�� �� ��B��A+G�� ��
��B��A+G�� �� (3)������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��
(3)������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��  ��
��(1)������ƫ�����ƣ�(2)��8Al + 3Fe3O4  9Fe+ 4Al2O3����3Fe + 4H2O
9Fe+ 4Al2O3����3Fe + 4H2O  Fe3O4 + 4H2����2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2����(3) MnO2+H2O2+2H��=== Mn2��+O2��+2H2O
Fe3O4 + 4H2����2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2����(3) MnO2+H2O2+2H��=== Mn2��+O2��+2H2O
 9Fe+ 4Al2O3����3Fe + 4H2O
9Fe+ 4Al2O3����3Fe + 4H2O  Fe3O4 + 4H2����2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2����(3) MnO2+H2O2+2H��=== Mn2��+O2��+2H2O
Fe3O4 + 4H2����2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2����(3) MnO2+H2O2+2H��=== Mn2��+O2��+2H2O��

��ϰ��ϵ�д�
�����Ŀ