��Ŀ����
��ҵ���������̿�������������Һ��ȡ����̣�����������Һ�Ʊ��ߴ�̼���̡���������淋Ĺ�����������(���̿����Ҫ�ɷ���MnO2�������й衢��������������������ؽ��������������)��
��1��һ���¶��£���������̡���Ҫ����ΪMnSO4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2��������2������Ҫ�ɷֵĻ�ѧʽΪ ��
��3�������ؽ�����ʱʹ��(NH4)2S����ʹ��Na2S��ԭ���� ��
��4������⡱ʱ�ö��Ե缫�������ĵ缫��ӦʽΪ ��
��5����50��̼�����õ��ߴ�̼���̣���Ӧ�����ӷ���ʽΪ ����50��̼����ʱ�������NH4HCO3�����ܵ�ԭ���ǣ�ʹMnSO4���ת��ΪMnCO3�� �� ��
��1��MnO2��SO2=MnSO4
��H2O��SO2 =H2SO3��MnO2��H2SO3=MnSO4��H2O(2��)
��2��Fe(OH)3��Al(OH)3(2��)
��3��Na2S��������յ�(NH4)2SO4��Na������(2��)
��4��2H2O��4e��=O2����4H����4OH����4e����O2����2H2O(2��)
��5��Mn2����2HCO3- MnCO3����CO2����H2O(2��)
MnCO3����CO2����H2O(2��)
NH4HCO3��H����Ӧ����ֹMnCO3�����ܽ���ʧ��NH4HCO3���ȷֽ���ʧ(2��)
�����������ᡰ��Ч�ȡ������� ��100%��90%(1��)
��100%��90%(1��)
�������������(1)�÷�Ӧ�ķ�Ӧ��Ϊ��������Ͷ������̲���Ϊ�����̣���2����Ӧ�������γ��ӣ�һ����������ȥ�������ӣ��������dz�ȥ�ؽ����õ�������Һ���ʵڶ���Ӧ��ȥ���������������������3��ʹ��Na2S���������������ӣ���4����������̺��������Һ�����������̣��������������ӷŵ����������4OH����4e����O2����2H2O����5���÷�Ӧ�ķ�Ӧ��Ϊ̼����狀������̣�������̼���̣�Mn2����2HCO3- MnCO3����CO2����H2O�����ʱ��Һ�в�����������ҺΪ���ԣ����ܽ�̼���Σ��ʼ������̼����立�Ӧ������Һ����ԣ���ֹMnCO3�����ܽ���ʧ��̼����鱗��ȶ������ֽ⣬�������Լ��ֲ��ֽ���ʧ��
MnCO3����CO2����H2O�����ʱ��Һ�в�����������ҺΪ���ԣ����ܽ�̼���Σ��ʼ������̼����立�Ӧ������Һ����ԣ���ֹMnCO3�����ܽ���ʧ��̼����鱗��ȶ������ֽ⣬�������Լ��ֲ��ֽ���ʧ��
���㣺���黯�������в���Ŀ�ġ�ԭ���й����⡣

����6����ɫ��Һ���Ҵ������ӡ�NaHCO3��Һ��AgNO3��Һ��KOH��Һ��������һһ���ֵ��Լ��ǣ�
A�����Ƽ���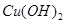 ����Һ ����Һ | B��FeCl3��Һ |
| C��BaCl2��Һ | D������KMnO4��Һ |
�ȼҵ��������Ļ�ѧ��ҵ֮һ�����IJ�ƷӦ�ù㷺����ش��������⣺
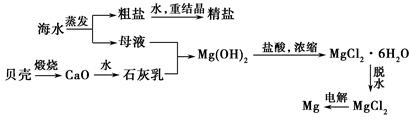
��1���ȼҵ�����õ��ʳ��ˮ���� Ϊ�����Ĺ�ҵ��ϵ��
��2�����ǰ��Ϊ��ȥʳ��ˮ�е�Mg2+��Ca2+��SO42-���������ӣ����������Լ���˳��������� (�����и��������)��
a��̼���ơ��������ơ��Ȼ��� b��̼���ơ��Ȼ�������������
c���������ơ�̼���ơ��Ȼ��� d���Ȼ������������ơ�̼����
��3�������ࡱ�ǵ��ʳ��ˮ�������γɵĹ�ҵ�����ϡ���ij����������������£�
| �ɷ� | NaCl | Mg(OH)2 | CaCO3 | BaSO4 | ���������� |
| ��������(��) | 15��20 | 15��20 | 5��10 | 30��40 | 10��15 |
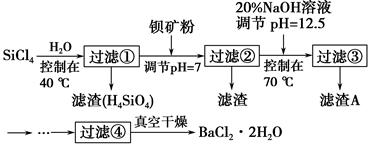
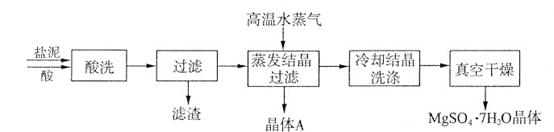
������������MgSO4��7H2O����Ĺ�����������ͼ��ʾ��
�ش��������⣺
����ϴ�����м������Ϊ ���������Ӧ�ʵ�����������pHΪ5���ң���Ӧ�¶���50�����ҡ���������ʹ֮��ַ�Ӧ����ʹMg(OH)2����ܽⲢת��ΪMgSO4���ڴ˹�����ͬʱ����CaSO4������̼��ƿ���ת��Ϊ����Ƶ�ԭ���� ��
�ڹ���������������Ҫ�ɷ�Ϊ ��
�۸���ͼ�����������ᾧ�������þ���A��Ҫ�ɷ�Ϊ ��

����ո���MgSO4��7H2O�����ԭ���� ��
CoCl2��6H2O��һ������Ӫ��ǿ������һ������ˮ�ܿ�(��Ҫ�ɷ�ΪCo2O3��Co(OH)3����������Fe2O3��Al2O3��MnO��)��ȡCoCl2��6H2O�Ĺ����������£�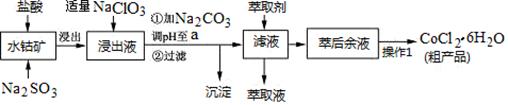
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���(��������Ũ��Ϊ��0.01mol/L)
| ������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��CoCl2��6H2O�۵�Ϊ86�棬������110~120��ʱ��ʧȥ�ᾧˮ������ˮ�Ȼ��ܡ�
��1��д������������Co2O3������Ӧ�����ӷ���ʽ________________________��
��2��д��NaClO3������Ӧ����Ҫ���ӷ���ʽ_____________________________������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽ_______________��
��3������Na2CO3��pH��a��,�������õ��ij����ɷ�Ϊ ��
��4��������1���а���3������ʵ�����������������_________��__________���ˡ��Ƶõ�CoCl2��6H2O�ں��ʱ���ѹ��ɵ�ԭ����__________________��
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ������Һ���м�����ȡ����Ŀ����_________����ʹ�õ����pH��Χ��________________��

A��2.0~2.5 B��3.0~3.5 C��4.0~4.5 D��5.0~5.5
��6��Ϊ�ⶨ�ֲ�Ʒ��CoCl2��6H2O��������ȡһ�������Ĵֲ�Ʒ����ˮ����������AgNO3��Һ�����ˡ�ϴ�ӣ���������ɺ����������ͨ�����㷢�ֲִ�Ʒ��CoCl2��6H2O��������������100������ԭ�������_____________________������һ�����ɣ�
�����(LiCoO2)����ӵ����һ��Ӧ�ù㷺�����͵�Դ��ʵ���ҳ������÷Ͼ����������ӵ�ػ�����������ͭ���ܡ��Ԫ�أ�ʵ��������£�
(1)����ݹ����У������ܽ�����ӷ���ʽΪ__________________________
(2)��ҺA�м���������Һ��ʹCoԪ����CoC2O4��2H2O������ʽ���������������Ʊ������ܼ��ܷ۵���Ҫԭ�ϡ��ڿ�����CoC2O4��2H2O���ȷֽ�ʧ�����ݼ��±����벹���������е��ȷֽⷽ��ʽ��
| ��� | �¶ȷ�Χ/�� | �ȷֽⷽ��ʽ | ����ʧ���� |
| �� | 120��220 | | 19.67% |
| �� | 280��310 | | 56.10% |
(3)����Li2CO3ʱ������Һ�����������ǣ���ʵ������ĽǶȸ������ֿ��ܵ�ԭ��_____________________________________________________________
(4)�������õ�FeCl3��Һ������ˮ�����Խ�����ӷ���ʽ�����侻ˮԭ��________________________________________________________