��Ŀ����
��������ҽҩ��ʯ����ҵ���й㷺��;����ͼ��ģ�ҵ�Ʊ��������Ʒ�����Ƶ����̣�
��֪��Br2���ӷ��������ɫ��Һ�壻���������ӷ�����ɫҺ�塣
�����������̻ش��������⣺
��1����Ӧ�Ң��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧ�Ң�ʹ�ñ�ˮ��Ŀ�� ��
��3������I������ ���������õ��IJ��������� ��
��4����Ӧ�Ң��м���Na2SO3��Ŀ���� ��
��5����ҵ�������Ƶõ���������е����Ļ�ɫ�����Ǽ�����ͬѧ�����ʵ�����̽����
�ټ�ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3����������֤���ü������õ��Լ�Ϊ ������������ɹ۲쵽������Ϊ ��
����ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ ��������֤���ü�������ķ���Ϊ ��
��1��SO2+Br2+2H2O=H2SO4+2HBr ��2����ֹBr2��HBr�ӷ�
��3������©�������������ձ��� ��4����ԭ��Ʒ�е�Br2
��5����KSCN��Һ����������KSCN��Һ���ɫ�����������к���Br2���ò�����պȡ�Ƶõ������ᣬ����ʪ�����KI��ֽ�ϱ��������ý�ͷ�ι�ȡ�Ƶõ����������Թ��У��μ�CCl4������ֹ���²�ʳȺ�ɫ����֤����Br2���Ի�ɫ��
���������������1����Ӧ�Ң���SO2��Br2�ڱ�ˮ�з�����Ӧ�Ļ�ѧ����ʽΪSO2+Br2+2H2O=H2SO4+2HBr����2����Ӧ�Ң�ʹ�ñ�ˮ��Ŀ����Ϊ�˷�ֹBr2��HBr�ӷ�����Ⱦ������Ӱ���������3������I�Ƿ��뻥�ܵķе㲻ͬ��Һ�����ʵIJ��������������������õ��IJ���������©�������������ձ�����4����Ӧ�Ң��м���Na2SO3��Ŀ����Ϊ������Ϊ��Ӧ��Br2��������ʵĴ��ȡ���5����ҵ�������Ƶõ���������е����Ļ�ɫ���ټ�ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3��������ķ�����ȡ��������Һ�������еμӼ���KSCN��Һ������ҵ��������KSCN��Һ���ɫ����֤������Fe3��������Ͱɺ���Fe3��������ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ����Br2������ķ�����������ǿ�����ԡ��ò�����պȡ�Ƶõ������ᣬ����ʪ�����KI��ֽ�ϱ�����Ҳ�������������л��ܼ��е��ܽ�ȴ�����ʡ��ý�ͷ�ι�ȡ�Ƶõ����������Թ��У��μ�CCl4������ֹ���²�ʳȺ�ɫ����֤����Br2���Ի�ɫ��
���㣺����SO2��Br2�����ʡ������ķ��뷽����������Fe3����Br2�ļ��鷽����֪ʶ��

�Ӻ�������ȡ���ʵ������У�������ȷ�IJ�����


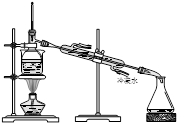
| A���������ճɻ� | B�����˺�I����Һ | C���ų���ı���Һ | D������Ⲣ���ձ� |
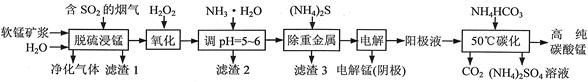
��ҵ����̼���̿�Ϊ��Ҫԭ������MnO2�Ĺ����������£�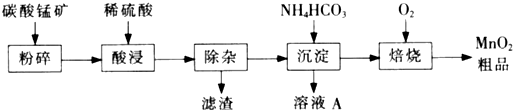 �й��������↑ʼ�����ͳ�����ȫ��pH���±���
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al��OH��2 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Pb��OH��2 | Mn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
���ʴ��������⣺
��1�����ǰ��̼���̿����������� ��
��2����������Һ�к���Mn2+�� SO42-������������Fe2+��Fe3+��A13+��Cu2+��Pb2+�ȣ�����ӹ������£�
�ټ���MnO2��Fe2+�����������ӷ�Ӧ����ʽΪ �� �ڼ���CaO����Һ��pH����5.2��6.0������ҪĿ���� ��
�ۼ���BaS����ȥCu2+��Pb2+���ټ���NaF��Һ����ȥ ��
��3������ҺA�л��յ���Ҫ������ �������ʳ��������ʡ���4��MnO2��Ʒ�к�������Mn3O4��������ϡ���ᴦ��������ת��ΪMnSO4��MnO2��Ȼ��������������Mn2+ת��ΪMnO2���Ƶ�����MnO2��д��Mn3O4��ϡ���ᷴӦ�Ļ�ѧ����ʽ ��
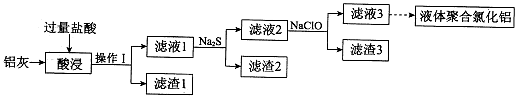
������ˮ������ҵ��ɰ�ǡ���֬��Ư����ɱ�������У���������(NaClO2)��������Ҫ�����á���ͼ�������������ƵĹ�������ͼ��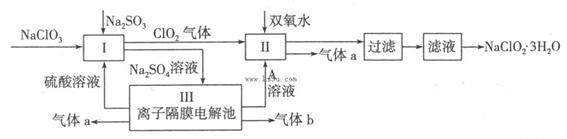
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
�ڳ����£�Ksp(FeS)=6��3��10-18��Ksp(CuS)=6��3��10-28��Ksp(PbS)=2��4 ��10-28
��1����ӦI�з�����Ӧ�����ӷ���ʽΪ ��
��2������Һ�еõ�NaClO2��3H2O������������������ (��д���)��
a������ b������Ũ�� c������ d����ȴ�ᾧ e������
��3��ӡȾ��ҵ������������(NaClO2)Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
| ���� | HClO2 | HF | H2CO3 | H2S |
| Ka��mol��L-1 | 1��10-2 | 6.3��10-4 | K1=4.30��10-7 K2=5.60��10-11 | K1=9.1��10-8 K2=l.1��10-12 |
�ٳ����£����ʵ���Ũ����ȵ�NaClO2��NaF��NaHCO3��Na2S������Һ��pH�ɴ�С��˳��Ϊ (�û�ѧʽ��ʾ)�������ȣ����ʵ���Ũ����ͬ��NaF��NaClO2����Һ�������������������Ĵ�С��ϵΪ�� (�ǰ�ߴ���ȡ����ߴ�)��
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij����� �������£������һ�����ӳ�����ȫʱ(������Ũ��Ϊ10-5mol��L-1)��ʱ��ϵ�е�S2-��Ũ��Ϊ ��
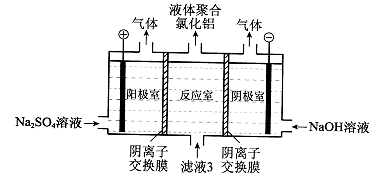
��4����װ������������a�ĵ缫��Ӧʽ ������������a�����Ϊ1��12L(��״��)����ת�Ƶ��ӵ����ʵ���Ϊ ��
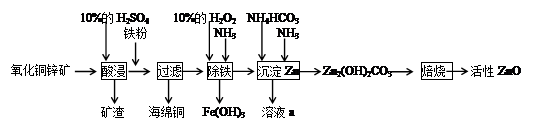
 2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4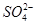 ��2H2O
��2H2O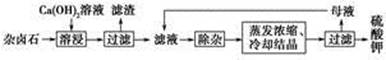

 ?
? CaCO3(s)��
CaCO3(s)��