��Ŀ����
16����2.0L�����ܱ������г���1.0mol PCl5�����¶�ΪTʱ�������·�ӦPCl5��g��?PCl3��g��+Cl2��g����H=+124kJ•mol-1����Ӧ�����вⶨ�IJ������ݼ��±���| ʱ��t/s | 0 | 50 | 150 | 250 | 350 |
| n��PCl3��/mol | 0 | 0.16 | 0.19 | 0.2 | 0.2 |
��1����Ӧ��ǰ50s��ƽ������v��PCl5��=0.0016mol/��L•s����
��2���¶�ΪTʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��=0.025��
��3��������Ӧ����ƽ��״̬ʱ��PCl3���������Ϊ16.7%��
Ҫ���ƽ��ʱPCl3������������ɲ�ȡ�Ĵ�ʩ��CD��
A���¶Ȳ��䣬ѹ�������������ѹǿ B��ʹ�ø�Ч����
C���¶Ⱥ�������䣬��СPCl5����ʼ�� D��������䣬��߷�Ӧ�¶�
��4�����¶�ΪTʱ������ʼʱ�������г���0.5mol PCl5��a mol Cl2ƽ��ʱPCl5��ת������Ϊ20%����a=0.1��
��5������ˮ�У����Ȼ�����ȫˮ�⣬�������ᣨH3PO4�����÷�Ӧ�Ļ�ѧ����ʽ��PCl5+4H2O=H3PO4+5HCl��
���� ��1���ɱ������ݿ�֪��50s�ڡ�n��PCl3��=0.16mol���ɷ���ʽ��֪��n��PCl5��=��n��PCl3��������v=$\frac{\frac{��n}{V}}{��t}$����v��PCl5����
��2���¶�ΪTʱ����2.0L�����ܱ������г���1.0mol PCl5��ƽ��ʱn��PCl3��=0.2mol����
PCl5��g��?PCl3��g��+Cl2��g��
��ʼ��mol/L����0.5 0 0
�仯��mol/L����0.1 0.1 0.1
ƽ�⣨mol/L����0.4 0.1 0.1
�ٸ���K=$\frac{c��PC{l}_{3}����c��C{l}_{2}��}{c��PC{l}_{5}��}$����ƽ�ⳣ����
��3��PCl3���������=$\frac{PC{l}_{3}���ʵ���}{������������ʵ���}$��100%��
Ҫ���ƽ��ʱPCl3������������ı�����ƽ�������ƶ��������ܼ�СPCl3��Ũ�ȣ�ע��ı�PCl5��Ũ�ȵ�ЧΪѹǿ�ı䣻
��4��ƽ��ʱPCl5��ת������Ϊ20%��ת����PCl5Ϊ0.5mol��20%=0.1mol����ʾ��ƽ��ʱ��������ʵ������ٸ���ƽ�ⳣ���з��̼�����
��5������ˮ�У����Ȼ�����ȫˮ�⣬�������ᣨH3PO4����������HCl��
��� �⣺��1���ɱ������ݿ�֪��50s�ڡ�n��PCl3��=0.16mol���ɷ���ʽ��֪��n��PCl5��=��n��PCl3��=0.16mol����v��PCl5��=$\frac{\frac{0.16mol}{2L}}{50s}$=0.0016mol/��L•s�����ʴ�Ϊ��0.0016mol/��L•s����
��2���¶�ΪTʱ����2.0L�����ܱ������г���1.0mol PCl5��ƽ��ʱn��PCl3��=0.2mol����
PCl5��g��?PCl3��g��+Cl2��g��
��ʼ��mol/L����0.5 0 0
�仯��mol/L����0.1 0.1 0.1
ƽ�⣨mol/L����0.4 0.1 0.1
��ƽ�ⳣ��K=$\frac{c��PC{l}_{3}����c��C{l}_{2}��}{c��PC{l}_{5}��}$=$\frac{0.1��0.1}{0.4}$=0.025��
�ʴ�Ϊ��0.025��
��3���ɣ�2���м������ݿ�֪��ƽ��ʱPCl3���������=$\frac{0.1mol/L}{0.6mol/L}$��100%=16.7%��
A���¶Ȳ��䣬ѹ�������������ѹǿ��ƽ�������ƶ���PCl3�����������С����A����
B��ʹ�ø�Ч��������Ӱ��ƽ���ƶ���PCl3������������䣬��B����
C���¶Ⱥ�������䣬��СPCl5����ʼ������ЧΪ����ѹǿ��ƽ�������ƶ���PCl3�������������C����
D��������䣬��߷�Ӧ�¶ȣ�����ӦΪ���ȷ�Ӧ��ƽ�������ƶ���PCl3�������������D��ȷ��
�ʴ�Ϊ��16.7%��CD��
��4�����¶�ΪTʱ������ʼʱ�������г���0.5mol PCl5��a mol Cl2��ƽ��ʱPCl5��ת������Ϊ20%����ת����PCl5Ϊ0.5mol��20%=0.1mol����Ӧ������У���
PCl5��g��?PCl3��g��+Cl2��g��
��ʼ��mol/L����0.25 0 0.5a
�仯��mol/L����0.05 0.05 0.05
ƽ�⣨mol/L����0.2 0.05 0.5a+0.05
��ƽ�ⳣ��K=$\frac{��0.5a+0.05����0.05}{0.2}$=0.025�����a=0.1
�ʴ�Ϊ��0.1��
��5������ˮ�У����Ȼ�����ȫˮ�⣬�������ᣨH3PO4����������HCl����Ӧ����ʽΪ��PCl5+4H2O=H3PO4+5HCl��
�ʴ�Ϊ��PCl5+4H2O=H3PO4+5HCl��
���� ���⿼�黯ѧƽ�������Ӱ�����ء���Ӧ���ʡ�ƽ�ⳣ������ȣ��Ѷ��еȣ���4����ע������ƽ������ǽ��м�����

 ��У����ϵ�д�
��У����ϵ�д�T1�¶��µIJ���ʵ������Ϊ
| t/s | 0 | 500 | 1000 | 1500 |
| C��N2O5��mol/L | 5.00 | 3.52 | 2.50 | 2.50 |
| A�� | 500s��N2O5�ֽ�����Ϊ2.96��10-3 mol/��L•s�� | |
| B�� | T1�¶��µ�ƽ�ⳣ��ΪK1=125��1000sʱת����Ϊ50% | |
| C�� | T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����K1��K2����T1��T2 | |
| D�� | ƽ��������������䣬�����������ѹ����ԭ����1/2������ƽ��ʱC��N2O5����5.00mol/L |
�仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=$\frac{c��CO����c��{H}_{2}O��}{c��C{O}_{2}����c��{H}_{2}��}$��
��2���÷�ӦΪ���ȷ�Ӧ������ȡ����ȡ�����
��3�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������BC��
a��������ѹǿ���� b����������� c��CO������
c��v����H2��=v����H2O�� d��c ��CO2��=c ��CO��
��4��830���£������ʵ�Ũ�ȹ�ϵ��c ��CO2��•c ��H2����c ��CO��•c ��H2O�������ʱ����Ӧ�������淴Ӧ���ʵĹ�ϵ��a��
a��v����v�� b��v��=v�� c��v����v�� d�����ж�
��5��830���£���2L�ܱ������У�����2mol CO2 ��2mol H2�����¶��·�Ӧ10���Ӵﵽƽ�⣬��ƽ�ⳣ��K=1.0����10������v ��H2��=0.05 mol/��L•min����ƽ��ʱCO2��ת����Ϊ50%��
 ����̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ���⣮
����̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ���⣮��1���õ绡���ϳɵĴ�������̼�ܳ����д�����̼�����������ʣ������ֿ������������������ᴿ������ɸ÷�Ӧ�Ļ�ѧ����ʽ�����ڷ���������ϵ����
��C+��KMnO4+��H2SO4=��CO2��+��MnSO4+��K2SO4+��6H2O
��2������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ
CO��g��+H2O��g��?CO2��g��+H2��g�����õ����¶������ݣ�
| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
������С������λ������ͬ����
��ʵ��2������ƽ�ⳣ��K=0.17���÷�ӦΪ���ȣ�����ȡ����ȡ�����Ӧ��
��3����֪�ڳ��³�ѹ�£�
��2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��g����H1=-1275.6kJ/mol
��2CO ��g��+O2��g���T2CO2��g����H2=-566.0kJ/mol
��H2O��g���TH2O��l����H3=-44.0kJ/mol
д���״�����ȫȼ������һ����̼����̬ˮ���Ȼ�ѧ����ʽ��CH3OH��l��+O2��g��=CO��g��+2H2O��g����H=-354.8KJ/mol��
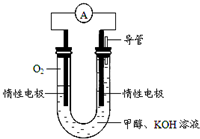
��4��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�ã�
�ٸõ�������ĵ缫��ӦʽΪ��O2+4e-+2H2O=4OH-���õ缫��ÿ����1.6g������ת�Ƶĵ�����Ϊ0.2mol��
�ڸõ�ع���ʱ����Һ�е�OH-����������������ƶ���
| A�� | Be+$\frac{1}{2}$O2�TBeO��H=-564.3kJ•mol-1 | |
| B�� | Be��s��+$\frac{1}{2}$O2��g���TBeO��s����H=+564.3kJ•mol-1 | |
| C�� | Be��s��+$\frac{1}{2}$O2��g���TBeO��s����H=-564.3kJ•mol-1 | |
| D�� | Be��s��+$\frac{1}{2}$O2��g���TBeO��g����H=-564.3kJ•mol-1 |
��1��ʵ���ã�8g�״��������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ�������������Ȼ�ѧ����ʽ��ȷ����C
A��$\frac{1}{4}$CH30H+$\frac{3}{8}$02=$\frac{1}{4}$C02+$\frac{1}{2}$H20��H=-113.5kJ•mol-1
B��$\frac{1}{4}$CH30H��l��+$\frac{3}{8}$02��g��=$\frac{1}{4}$C02��g��+$\frac{1}{2}$H20��l����H=+113.5kJ•mol-1
C��2CH30H��l��+302��g��=2C02��g��+4H20��l����H=-908kJ•mol-1
D��2CH30H��l��+302��g��=2C02��g��+4H20��l����H=+908kJ•mol-1
��2������̬��̬ԭ���γ�1mol��ѧ���ͷŵ���������м��ܣ��ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̣��ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
| ��ѧ�� | H-H | N-H | N��N |
| ����/kJ•mol-1 | 436 | 391 | 945 |
��3���ɽ��ʯ��TiO2���Ʊ�����Ti���漰�IJ���Ϊ��TiO2-��TiCl4$��_{800�棬Ar}^{Mg}$Ti
��֪����C��s��+O2��g���TCO2��g����H=-393.5kJ•mol-1
��2CO��g��+O2��g���T2CO2��g����H=-566kJ•mol-1
��TiO2��s��+2Cl2��g���TTiCl4��s��+O2��g����H=+141kJ•mol-1
��TiO2��s��+2Cl2��g��+2C��s���TTiCl4��s��+2CO��g���ġ�H=-80kJ•mol-1��
| A�� | �����ȼ���ȡ�H=-890.3kJ/mol�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-890.3 kJ/mol | |
| B�� | һ�������£���0.5 mol N2��1.5 molH2�����ܱ������г�ַ�Ӧ����NH3����19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-38.6 kJ/mol | |
| C�� | ��101kPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8 kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2��g��+O2��g���T2H2O��l����H=-571.6 kJ/mol | |
| D�� | HCl��NaOH��Ӧ���к��ȡ�H=-57.3 kJ/mol����H2SO4��Ba��OH��2��Ӧ���к��ȡ�H=2����-57.3��kJ/mol |
| A�� | ���������˵��¶ȷ�Χʱ�Ĵ�Ч������ | |
| B�� | ʹ�ô����������������Ӱٷ������ӿ췴Ӧ���� | |
| C�� | �����ڻ�ѧ��Ӧǰ�������ͻ�ѧ���ʲ�������Ϊ�������μӻ�ѧ��Ӧ | |
| D�� | п�����ᷴӦʱ�����뼸������ͭ��Һ�ɼӿ췴Ӧ���ʣ�������ͭ�������� |