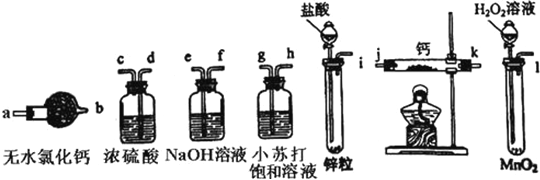
��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣��ش���ص����⡣
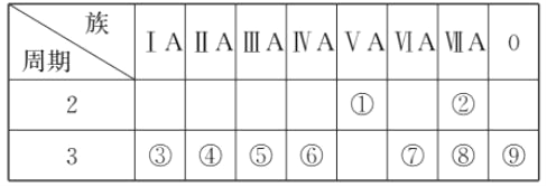
��1��д���ܵ�Ԫ�ط���________��
��2������ЩԪ���У�����õĽ���Ԫ����ˮ��Ӧ�����ӷ���ʽ��___________________________��
��3������ЩԪ���У�����������ˮ����������ǿ����________(����Ӧ��ѧʽ����ͬ)��������ǿ����________��
��4����ЩԪ����(������)��ԭ�Ӱ뾶��С����________(��Ԫ�ط��ţ���ͬ)��ԭ�Ӱ뾶������________��
��5���ڵĵ�����۵�����������ˮ�������Һ��Ӧ�������֮һ��OX2 �� (O��X�ֱ��ʾ���͢ڵ�Ԫ�ط��ţ���OX2�����û�ѧʽ)���÷�Ӧ�����ӷ���ʽΪ(����ʽ���þ���Ԫ�ط��ű�ʾ) _____________________________________________________ ��
���𰸡�Mg 2Na+ 2H2O = 2Na+ +2OH�� + H2�� HClO4 NaOH F Na 2F2 + 2OH- = OF2 + 2F- + H2O
��������
����Ԫ����Ԫ�����ڱ��е�λ�ü�Ԫ�������ɷ�����𣬸������ʵĻ�ѧ������д���ӷ���ʽ��
��1���ܺ�Ԫ���ڵ�������IIA�壬��12��Ԫ��Mg��
�ʴ�Ϊ��Mg��
��2������õĽ���λ��IA�壬�ǽ����ƣ���ˮ��Ӧ�����ӷ���ʽ��2Na+ 2H2O = 2Na++2OH�� + H2����
�ʴ�Ϊ��2Na+ 2H2O = 2Na+ +2OH�� + H2����
��3���ǽ�����Խǿ������������ˮ��������Խǿ��������Խǿ������������ˮ�������Խǿ�����ԣ�����ЩԪ���У�����������ˮ����������ǿ����HClO4��������ǿ����NaOH��
�ʴ𰸣�HClO4��NaOH��
��4��ͬ����Ԫ�غ˵��Խ�뾶ԽС��ͬ����Ԫ�أ��˵��Խ��뾶Խ��������ЩԪ���У�ԭ�Ӱ뾶��С����F��ԭ�Ӱ뾶������Na��
�ʴ�Ϊ��F��Na ��
��5����ΪF���۵�����������ˮ����Ϊ�������ƣ���Ӧ�����ӷ���ʽΪ2F2 + 2OH- = OF2 + 2F- + H2O���ʴ�Ϊ2F2 + 2OH- = OF2 + 2F- + H2O��

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�