��Ŀ����
A��B��C����Ԫ�����ڱ��еĶ����ڷǽ���Ԫ�أ����ǵĺ˵������������Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2����Bԭ�ӵ������p����ĵ���Ϊ�����ṹ��C�ǵؿ��к�������Ԫ�ء�D��E�ǵ�������Ԫ�أ�Dԭ�Ӻ���������������1�����ӣ����������Ӿ�������Eԭ�Ӻ���δ�ɶԵ�������ͬ��������ࡣ���ö�Ӧ��Ԫ�ط��Ż�ѧʽ��գ�
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ �� A��C���⻯��е��С��ϵΪ������������������ԭ��Ϊ������������������������������������������������
����������������������������������������������������������������������������
��2��D��E��ԭ�ӻ��ȷֱ�Ϊ340 kJ��mol-1��400kJ��mol-1�������ǵ��۵㣺D E(�����������������="��" )��
��3������A2B2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�Ϊ�Ǽ��Է��ӣ�ÿ��ԭ������������������˵��ӣ���ṹʽΪ_____________��1mol�÷����к��� ������ĿΪ ��
������ĿΪ ��
��4����̬��ԭ�ӵ���Χ�����Ų�ʽΪ ��EO2Cl2�۵㣺��96 .5�棬�е㣺117�棬���̬EO2Cl2���� ���塣
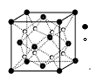
��5��D���⻯��ľ���ṹ��ͼ��ʾ���仯ѧʽ�� ��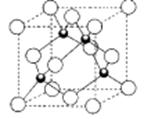
��C��O��N��1�֣���H2O��CH4����1�֣� H2O���Ӽ���ڷ��Ӽ��������H2OΪ���Է��ӣ�2�֣���
��<��1�֣���N��C��C��N��2�֣�4NA����2.408��1024����2�֣�
��3d54s1��1�֣� ���ӣ�1�֣�
��CuH��2�֣�
�������������Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2������A�ĵ����Ų�Ϊ1s22s1��1s22s22p2ΪLi��C��Bԭ�ӵ������p����ĵ���Ϊ�����ṹ����s2p3�ṹ��ΪN��P��C�ǵؿ��к�������Ԫ��ΪO����A��B��C����Ԫ�����ڱ��еĶ����ڷǽ���Ԫ�أ����ǵĺ˵������������ABC�ֱ�ΪC��N��O��D��E�ǵ�������Ԫ�أ�Dԭ�Ӻ���������������1�����ӣ����������Ӿ������������Ų�Ϊ[Ar]3d104s1��ΪCu��Eԭ�Ӻ���δ�ɶԵ�������ͬ��������࣬ӦΪ3d54s1��ΪCrԪ�أ��ʣ�1��A��B��C�ĵ�һ��������С�����˳��ΪC��O��N��A��C���⻯��е��С��ϵΪH2O��CH4����ΪH2O���Ӽ���ڷ��Ӽ��������H2OΪ���Է��ӣ���2��D��ԭ�ӻ��ȣ�E��ԭ�ӻ��ȹ��۵㣺D ��E����3������A2B2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�Ϊ�Ǽ��Է��ӣ�ÿ��ԭ������������������˵��ӣ���ṹʽΪN��C��C��N�� ������ĿΪ4����1mol�÷����к���
������ĿΪ4����1mol�÷����к��� ������ĿΪ4NA����4����̬��ԭ�ӵ���Χ�����Ų�ʽΪ3d54s1�����������ʣ���֪��̬CrO2Cl2���ڷ��Ӿ��壻��5�����ݾ����ṹ��֪����Hԭ�Ӹ���Ϊ4��Cuԭ�Ӹ���Ϊ8��1/8+6��1/2=4���ʻ�ѧʽΪCuH��
������ĿΪ4NA����4����̬��ԭ�ӵ���Χ�����Ų�ʽΪ3d54s1�����������ʣ���֪��̬CrO2Cl2���ڷ��Ӿ��壻��5�����ݾ����ṹ��֪����Hԭ�Ӹ���Ϊ4��Cuԭ�Ӹ���Ϊ8��1/8+6��1/2=4���ʻ�ѧʽΪCuH��
���㣺����Ԫ�ص��ƶϡ�ԭ�Ӻ�������Ų��������ܡ����ۼ����͡�����֪ʶ�����ݡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�(15��)
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B ��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
��1��A�����ڱ��е�λ���� ��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ ��
��2��B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ ����ˮ��Һ��F���ʷ�Ӧ�Ļ�ѧ����ʽΪ ���ڲ����м�������KI����Ӧ�����CC14������ ������ ɫ��
��3������ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ����A�Ķ�Ԫ���ӻ����� |
| b | ���зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1:1 |
| c | ��ѧ���ΪBDF2 |
| d | ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
d�ľ��������� ��
��4����A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ�� �����ɾ��п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ ��
(9��)Ԫ����������ָ������ѧϰԪ�ؼ��仯����֪ʶ����Ҫ���ߡ���֪����Ԫ�أ�����Po���IJ���֪ʶ���±���ʾ��
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵㣨�棩 | -218.4 | 113 | | 450 |
| ���ʷе㣨�棩 | -183 | 444.6 | 685 | 1390 |
| Ԫ����Ҫ���ϼ� | -2 | -2,+4,+6 | -2,+4,+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
��1�����������۵㷶Χ������________________��
��2��Ԫ���ڵ���Ҫ���ϼۿ�����________________��
��3���������ڵ��⻯��ˮ��Һ��������ǿ������˳����________________(�û�ѧʽ��ʾ)��
��4���������н�ǿ��__________��������ԡ���ԭ�ԡ��������¶���ڿ����г��ڱ����ױ��ʣ�����ܷ�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
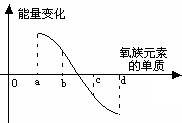
��5����ͼ��ʾΪ����Ԫ�ص�����H2��Ӧ�����е������仯ʾ��ͼ������a��b��c��d�ֱ��ʾ����Ԫ����ijһԪ�صĵ��ʣ�������Ϊ��ͬ���ʵ����ĵ�����H2��Ӧ�����е������仯�������仯��0��ʾ���ȣ������仯��0��ʾ���ȣ�����b����___________ _____�� d���� (��д��������)��
��16�֣��ȵ���ԭ���Ļ����۵��ǣ�ԭ������ͬ�Ҽ۵���������ȵķ��ӻ����Ӿ�����ͬ�Ļ�ѧ�����ͺͿռ乹�ͣ�����Ϊ�ȵ����塣�ȵ�����Ľṹ���ƣ���������������磺N2��CO��C22����CN��Ϊ�ȵ����塣
��1����֪CaC2Ϊ���ӻ������CaC2�ĵ���ʽΪ ��
��2���۱�ϩ���׳�������ë���ɱ�ϩ�����CH2=CH��CN���ۺϷ�Ӧ���ɣ���CH2=CH��CN��Cԭ�ӵ��ӻ���ʽΪ �������ЦҼ��ͦм���֮��Ϊ ��
��3��CO������ɽ���ԭ��M�γ������M(CO)n ��������������ԭ�Ӽ۵���������λ���ṩ��������֮��Ϊ18����MΪFe����n= ��
��4��CO��N2�Ľṹ���ƣ������к��й����������ɱ�ʾΪC��O ���±������ߵļ������ݣ���λ��kJ��mol��1��
| | C��O | C=O | C��O |
| CO | 357.7 | 798.9 | 1071.9 |
| | N��N | N=N | N��N |
| N2 | 154.8 | 418.4 | 941.7 |
��5��Fe3+��Fe2+��Co3+��Co2+������CN-�γ���������������Һ�м������KCN��Һ����������ɫ����K4[Fe(CN)6]������������Һ����ͨ���������������ɫ����K3[Fe(CN)6]����K4[Fe(CN)6]��K3[Fe(CN)6]�����ж������ڵ������������� �������ţ�
��A�����Ӽ����� B�����ۼ����� C������������D����λ������ E�����»���
��6��д����NO3����Ϊ�ȵ�����ķ��� ��д��һ�֣���