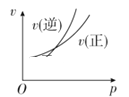
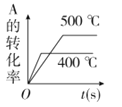
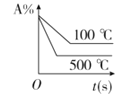
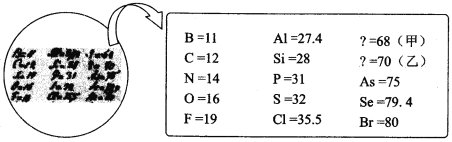
��Ŀ����
����Ŀ�������£�ij�ݻ��̶����ܱ������ɿ��ƶ��Ļ�������A��B���ң��ֱ���A��B���ҳ���H2��O2�Ļ�������1 mol��������ʱ������λ����ͼ��ʾ��
(1)A�һ����������ʵ���Ϊ________����������Ϊ________��
(2)ʵ����A�һ�����������Ϊ34 g����û��������ܶ���ͬ��ͬѹ�����º����ܶȵ�________��������A��H2��O2�Ļ�������ȼ�������ָ�ԭ�¶Ⱥ����ջ���ͣ����λ����________�̶ȣ�����������ѹǿ�뷴Ӧǰ����ѹǿ֮��Ϊ________��
���𰸡�2mol 44.8L 4.25 2 1��2
��������
(1)ͬ��ͬѹ�£���������ʵ���֮�ȵ���������֮�ȣ�����n=![]() �������������
�������������
(2)����A���л�������ƽ��Ħ��������ͬ��ͬѹ��������ܶ�֮�ȵ�����Ħ������֮�ȣ������������������ʵ����з��̼����������������ʵ��������ݷ���ʽ���㷴Ӧ��ʣ���������ʵ������ָ�ԭ�¶Ⱥ�����������ѹǿ��ȣ����֮�ȵ��������ʵ���֮�ȣ�����ȷ������ͣ����λ�ã�B���п������ʵ������䡢�¶Ȳ��䣬��Ӧǰ��ѹǿ֮���뷴Ӧǰ������ɷ��ȡ�
(1)A��B����ѹǿ���¶���ͬ����������ʵ���֮�ȵ���������֮�ȣ���A�����������ʵ���Ϊ1mol��![]() =2mol�������ڱ�״�������ΪV=n��Vm=2mol��22.4L/mol=44.8L��
=2mol�������ڱ�״�������ΪV=n��Vm=2mol��22.4L/mol=44.8L��
(2)A���л�������ƽ��Ħ������ΪM=![]() =17g/mol��ͬ��ͬѹ��������ܶ�֮�ȵ�����Ħ������֮�ȣ����Ըû��������ܶ���ͬ��ͬѹ�����º����ܶȵ�D=
=17g/mol��ͬ��ͬѹ��������ܶ�֮�ȵ�����Ħ������֮�ȣ����Ըû��������ܶ���ͬ��ͬѹ�����º����ܶȵ�D=![]() =4.25����
=4.25����
��A�����������ʵ���Ϊxmol���������ʵ���Ϊymol����x+y=2��2x+32y=34�����x=1��y=1�����ݷ�Ӧ����ʽ2H2+O2![]() 2H2O�ж��߷�Ӧ�����ʵ�����ϵ��֪O2��������Ӧ��ʣ��O2�����ʵ�����0.5mol���ָ�ԭ�¶Ⱥ�����������ѹǿ��ȣ����֮�ȵ��������ʵ���֮�ȣ�����A��B���ҵ����֮��Ϊ0.5mol��1mol=1��2�������ͣ����2�̶ȴ���
2H2O�ж��߷�Ӧ�����ʵ�����ϵ��֪O2��������Ӧ��ʣ��O2�����ʵ�����0.5mol���ָ�ԭ�¶Ⱥ�����������ѹǿ��ȣ����֮�ȵ��������ʵ���֮�ȣ�����A��B���ҵ����֮��Ϊ0.5mol��1mol=1��2�������ͣ����2�̶ȴ���
A���п������ʵ������䡢�¶Ȳ��䣬��Ӧǰ��ѹǿ֮���뷴Ӧǰ������ɷ��ȣ�������������ѹǿ�뷴Ӧǰ����ѹǿ֮��Ϊ2��4=1��2��

����Ŀ�����ܱ���Ϊ��߷��w���������Դ���������ʹ���������������õ���Ҫ�о�������ش��������⣺
��.������A(H3BNH3)��һ��DZ�ڵĴ�����ϣ�������Ԫ��״����(HB=NH)3ͨ���� �·�Ӧ�Ƶ�:3CH4+2 (HB=NH) 3+6H2O=3CO2+6H3BNH3
(1)��̬Bԭ�ӵļ۵����Ų�ʽΪ___________��B��C�� N�� O��һ�������ɴ�С��˳��Ϊ_________��CH4��H2O��CO2�ļ��ǰ����ɴ�С��˳������Ϊ___________��
(2)��(HB=NH)3����Ϊ�ȵ�������л�����Ϊ___________(�����ʽ)��
��.�����İ�ȫ���������������Ӧ�õĹؼ���
(1)ӡ�����³�Ƚ���ѧ������ĵ�Datta��Pati���˽���ADF������һ���� �ͻ�ϩ�ഢ�����(C16S8)������峣������۽Ƕ�֤�����ֲ��ϵķ��ӳ�ƽ��ṹ(��ͼ)��ÿ���ӻ�ƽ������������������10��H2���ӡ�

��C16S8������Cԭ�Ӻ�Sԭ�ӵ��ӻ�������ͷֱ�Ϊ___________��
����ؼ������������ʾ��
��ѧ�� | C-S | C=S | C16S8��̼��� |
����/pm | 181 | 155 | 176 |
�ӱ������ݿ�����C16S8��̼�����������C��S����C=S��֮�䣬ԭ�����______________________��
��C16S8��H2�������������___________��
(2)�д���ܵ�ͭ�Ͻ�������������ܶѻ��ṹ��������Cuԭ��λ�����ģ�Ag ԭ��λ�ڶ��㣬��ԭ�ӿɽ�����Cuԭ����Agԭ�ӹ��ɵ��������϶�С��þ��崢���ľ����ṹ��CaF2(��ͼ)���ƣ��þ��崢���Ļ�ѧʽΪ___________��


(3)MgH2�ǽ����⻯�ﴢ����ϣ��侧������ͼ��ʾ���þ�����ܶ�Ϊagcm-3���������Ϊ___________cm3(�ú�a��NA�Ĵ���ʽ��ʾ��NA��ʾ�����ӵ�������ֵ)��