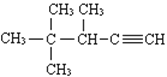
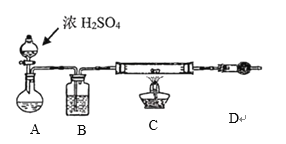
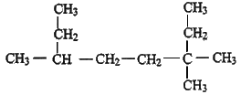��Ŀ����
����Ŀ��ʵ������Ҫ0.1mol/LNaOH��Һ450ml��0.5mol/L������Һ500ml��������������Һ����������ش��������⣺
��1����ͼ��ʾ�������У�������Һ�϶�����Ҫ����________������ţ�������������Һ�����õ��IJ���������________�����������ƣ���
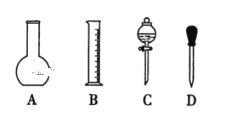
��2������0.1mol/L��NaOH��Һ�IJ����������£���ȷ��˳����_______��
�ٰѳ����õ�NaOH�������С�ձ��У�������������ˮ�ܽ⣻
������������ˮϴ���ձ��Ͳ�����2~ 3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ�У�������ҡ�ȣ�
�ۼ���������ƿ�м�����ˮ��Һ���̶���1~ 2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ�İ�Һ����̶������У�
�ܰѢ�������Һ��ȴ�����£���С��ת��һ���ݻ�������ƿ�У�
�ݽ�����ƿƿ�����������ҡ�ȡ�
��3�����ݼ��㣬��������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ�����������ҺŨ��________0.1mol/L��������������С������������������ͬ��������δ����Һ��ȴ�Ͷ��ݣ���������ҺŨ��________0.1mol/L�����ƺú��ֳ���ʱ���õ�������ƽ������������________0.1mol/L��
���𰸡�A C 500ml����ƿ�����������ձ� �٢ܢڢۢ� 2.0 ���� ���� ����
��������
��1�����Ʋ����м��㡢��������ȡ�����ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ��������ݲ����ж���ѡ������
��2������ʵ������ʵ�鲽���г������ܽ⡢��Һ��ϴ�ӡ������Լ�ҡ�ȵȲ�����
��3������n=cV��m=nM�����������Ƶ�������
����c=![]() ���
���
��1�����Ʋ����м��㡢��������ȡ�����ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����������Ϊ���壬һ����������ƽ��������ҩ��ȡ��ҩƷ��������ΪҺ�壬һ������Ͳ��ȡ��Ȼ�����ձ����ܽ⣬��ȴ��ת�Ƶ���Ӧ��������ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ����Բ���Ҫ��������AC������Ҫ��������500mL����ƿ�����������ձ���
��2������ʵ������ʵ�鲽���г������ܽ⡢��Һ��ϴ�ӡ������Լ�ҡ�ȵȲ���������ȷ��˳���ǣ��٢ܢڢۢݣ�
��3��������450mL������ƿ����ѡ��500mL������ƿ����Ҫ�������Ƶ����ʵ���n=cV=0.50mol/L��0.5L=0.25mol������Ϊm=nM=0.25mol��40g/mol=2.0g��
��ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ������������ҺŨ����Ӱ�죬����0.1mol/L��
����δ����Һ��ȴ�Ͷ��ݣ���ȴ����Һ�������С����������ҺŨ�ȴ���0.1mol/L��
���ƺú��ֳ���ʱ���õ�������ƽ�����������ˣ����������Ĺ�������ƫ��������Һ��Ũ��ƫ�������0.1mol/L��