ЬтФПФкШн
ЁОЬтФПЁПФГЮоЩЋЭИУїШмвКПЩФмКЌгаЯТСаРызгЃКK+ЁЂAl3+ЁЂFe3+ЁЂBa2+ЁЂNO3ЃЁЂSO42ЃЁЂHCO3ЃЁЂClЃЃЌШЁИУШмвКНјааШчЯТЪЕбщЃК
Ђй гУРЖЩЋЪЏШяЪджНМьВтИУШмвКЃЌЪджНЯдКьЩЋЃЛ
Ђк ШЁШмвКЩйаэЃЌМгШыЭЦЌКЭЯЁСђЫсЙВШШЃЌВњЩњЮоЩЋЦјЬх,ИУЦјЬхгіПеЦјСЂМДБфЮЊКьзиЩЋ
Ђл ШЁШмвКЩйаэЃЌМгШыАБЫЎгаАзЩЋГСЕэЩњГЩЃЌМЬајМгШыЙ§СПАБЫЎЃЌГСЕэВЛЯћЪЇЃЛ
Ђм ШЁШмвКЩйаэЃЌЕЮШыТШЛЏБЕШмвКВњЩњАзЩЋГСЕэЃЛ
Ђн ШЁЪЕбщ Ђм КѓЕФГЮЧхШмвКЃЌЕЮШыЯѕЫсвјШмвКВњЩњАзЩЋГСЕэЃЌдйМгШыЙ§СПЕФЯЁЯѕЫсЃЌГСЕэВЛЯћЪЇЁЃ ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉИљОнЩЯЪіЪЕбщХаЖЯдШмвКжаПЯЖЈДцдкЕФРызгЪЧ______________ЃЌПЯЖЈВЛДцдкЕФРызгЪЧ__________ЁЃ
ЃЈ2ЃЉаДГіЪЕбщЂкЕФРызгЗНГЬЪНЃК______________________
ЁОД№АИЁПAl3+ЁЂNO3-ЁЂSO42- Fe3+ЁЂBa2+ЁЂHCO3- 3CuЃЋ8H+ЃЋ2NO3- ЃН 3Cu2+ЃЋ2NO![]() ЃЋ4H2O
ЃЋ4H2O
ЁОНтЮіЁП
ИУШмвКЮоЩЋЃЌдђВЛДцдкFe3+ЃЛ
ЂйгУРЖЩЋЪЏШяЪджНМьВтИУШмвКЃЌЪджНЯдКьЩЋЃЌЫЕУїШмвКГЪЫсадЃЌдђвЛЖЈВЛДцдкHCO![]() ЃЌ
ЃЌ
ЂкШЁШмвКЩйаэЃЌМгШыЭЦЌКЭЯЁСђЫсЙВШШЃЌВњЩњЮоЩЋЦјЬхЃЌИУЦјЬхгіПеЦјСЂМДБфЮЊКьзиЩЋЃЌИУЦјЬхЮЊNOЃЌЫЕУїШмвКжаДцдкNO![]() ЃЛ
ЃЛ
ЂлШЁШмвКЩйаэЃЌМгШыАБЫЎгаАзЩЋГСЕэЩњГЩЃЌМЬајМгШыЙ§СПАБЫЎЃЌГСЕэВЛЯћЪЇЃЌЫЕУїКЌгаAl3+РызгЃЛ
ЂмШЁШмвКЩйаэЃЌЕЮШыТШЛЏБЕШмвКВњЩњАзЩЋГСЕэЃЌИУГСЕэЮЊСђЫсБЕГСЕэЃЌЫЕУїКЌгаSO![]() ЃЌдђвЛЖЈВЛЛсКЌгаBa2+ЃЛ
ЃЌдђвЛЖЈВЛЛсКЌгаBa2+ЃЛ
ЂнШЁЪЕбщЂмКѓЕФГЮЧхШмвКЃЌЕЮШыЯѕЫсвјШмвКВњЩњАзЩЋГСЕэЃЌдйМгШыЙ§СПЕФЯЁЯѕЫсЃЌГСЕэВЛЯћЪЇЃЌВЛФмжЄУїЪЧЗёКЌгаCl-РызгЃЌвђЂмжаМгШыТШЛЏБЕЃЌв§ШыСЫТШРызгЁЃ
ЃЈ1ЃЉИљОнЗжЮіЃЌдШмвКжаПЯЖЈДцдкЕФРызгЪЧAl3+ЁЂNO3-ЁЂSO42-ЃЌПЯЖЈВЛДцдкЕФРызгЪЧFe3+ЁЂBa2+ЁЂHCO3-ЃЛ
ЃЈ2ЃЉЂкЗДгІЮЊН№ЪєЭдкЫсадЬѕМўЯТгыЯѕЫсИљРызгЗЂЩњбѕЛЏЛЙдЗДгІЩњГЩЭРызгКЭвЛбѕЛЏЕЊЦјЬхЃЌЗДгІЕФРызгЗНГЬЪНЮЊ3Cu+8H++2NO![]() =3Cu2++2NOЁќ+4H2OЁЃ
=3Cu2++2NOЁќ+4H2OЁЃ

 вЛБОКУЬтПкЫуЬтПЈЯЕСаД№АИ
вЛБОКУЬтПкЫуЬтПЈЯЕСаД№АИЁОЬтФПЁПЯжЪЙгУЫсМюжаКЭЕЮЖЈЗЈВтЖЈЪаЪлАзДзЕФзмЫсСП(g/100mL)
ЂёЃЎЪЕбщВНжш
(1)ХфжЦ100mLД§ВтАзДзШмвКЃКгУ______![]() ЬювЧЦїУћГЦ
ЬювЧЦїУћГЦ![]() СПШЁ10.00mLЪаЪлАзДзЃЌдкЩеБжагУЫЎЯЁЪЭКѓзЊвЦЕН______
СПШЁ10.00mLЪаЪлАзДзЃЌдкЩеБжагУЫЎЯЁЪЭКѓзЊвЦЕН______![]() ЬювЧЦїШнСПЁЂУћГЦ
ЬювЧЦїШнСПЁЂУћГЦ![]() жаЖЈШнЃЌвЁдШМДЕУД§ВтАзДзШмвКЃЎ
жаЖЈШнЃЌвЁдШМДЕУД§ВтАзДзШмвКЃЎ
(2)гУЫсЪНЕЮЖЈЙмШЁД§ВтАзДзШмвК20.00mLгкзЖаЮЦПжаЃЌЯђЦфжаЕЮМг2ЕЮ_____зїжИЪОМСЃЎ
(3)ЖСШЁЪЂзА0.1000mol/LNaOHШмвКЕФМюЪНЕЮЖЈЙмЕФГѕЪМЖСЪ§:ШчЙћвКУцЮЛжУШчЭМЫљЪОЃЌдђДЫЪБЕФЖСЪ§ЮЊ______mLЃЎ
![]()
(4)ЕЮЖЈЃКЕБ________________________________________ЪБЃЌЭЃжЙЕЮЖЈЃЌВЂМЧТМNaOHШмвКЕФжеЖСЪ§ЃЌжиИДЕЮЖЈ3ДЮЁЃ
ЂђЃЎЪЕбщМЧТМ
ЕЮЖЈДЮЪ§ЪЕбщЪ§Он(mL) | 1 | 2 | 3 | 4 |
V(бљЦЗ) |
|
|
|
|
V(NaOH)(ЯћКФ) |
|
|
|
|
ЂѓЃЎЪ§ОнДІРэгыЬжТл
(5)МзЭЌбЇдкДІРэЪ§ОнЪБМЦЫуЕУЃКЦНОљЯћКФЕФNaOHШмвКЕФЬхЛ§ЃКV=![]() =15.24mLжИГіЫћЕФМЦЫуЕФВЛКЯРэжЎДІЃК_________________________________________________ЁЃ
=15.24mLжИГіЫћЕФМЦЫуЕФВЛКЯРэжЎДІЃК_________________________________________________ЁЃ
бЁШЁе§ШЗЪ§ОнЃЌПЩЕУ![]() ЪаЪлАзДз
ЪаЪлАзДз![]() ____mol/LЃЛЪаЪлАзДззмЫсСП
____mol/LЃЛЪаЪлАзДззмЫсСП![]() ____g/100mLЁЃ
____g/100mLЁЃ
(6)ввЭЌбЇзаЯИбаОПСЫИУЦЗХЦАзДзЕФБъЧЉЃЌЗЂЯжЦфжаЛЙКЌгаБНМзЫсФЦзїЮЊЪГЦЗЬэМгМСЃЌЫћЯыгУзЪСЯЗЈбщжЄДзЫсгыБНМзЫсФЦВЛЛсЗЂЩњРызгЛЅЛЛЗДгІЃЌашВщевдквЛЖЈЮТЖШЯТЕФДзЫсгыБНМзЫсЕФ________(ЬюБъКХ)ЃЎ
AЃЎpHBЃЎЕчРыЖШCЃЎЕчРыГЃЪ§DЃЎШмНтЖШ
(7)дкБОЪЕбщЕФЕЮЖЈЙ§ГЬжаЃЌЯТСаВйзїЛсЪЙЪЕбщНсЙћЦЋДѓЕФЪЧ______(ЬюБъКХ)ЃЎ
AЃЎМюЪНЕЮЖЈЙмдкгУеєСѓЫЎЯДОЛКѓЃЌЮДгУБъзМNaOHШмвКШѓЯД
BЃЎМюЪНЕЮЖЈЙмЕФМтзьдкЕЮЖЈЧАгаЦјХнЃЌЕЮЖЈКѓЦјХнЯћЪЇ
CЃЎзЖаЮЦПжаМгШыД§ВтАзДзШмвККѓЃЌдйМгЩйСПЫЎ
DЃЎзЖаЮЦПдкЕЮЖЈЪБОчСввЁЖЏЃЌгаЩйСПвКЬхНІГіЃЎ
ЁОЬтФПЁПУРЙњBayЕШЙЄГЇЪЙгУЪЏгЭШШСбНтЕФИБВњЮяМзЭщРДжЦШЁЧтЦјЃЌЦфЩњВњСїГЬШчЭМЫљЪОЃК
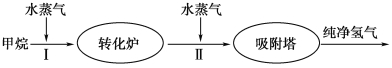
(1)ДЫСїГЬЕФЕкЂђВНЗДгІЮЊЃКCO(g)ЃЋH2O(g) ![]() H2(g)ЃЋCO2(g)ЃЌИУЗДгІЕФЛЏбЇЦНКтГЃЪ§БэДяЪНЮЊKЃН____________ЃЛЗДгІЕФЦНКтГЃЪ§ЫцЮТЖШЕФБфЛЏШчЯТБэЫљЪОЁЃ
H2(g)ЃЋCO2(g)ЃЌИУЗДгІЕФЛЏбЇЦНКтГЃЪ§БэДяЪНЮЊKЃН____________ЃЛЗДгІЕФЦНКтГЃЪ§ЫцЮТЖШЕФБфЛЏШчЯТБэЫљЪОЁЃ
ЮТЖШ/Ёц | 400 | 500 | 830 | 1 000 |
ЦНКтГЃЪ§K | 10 | 9 | 1 | 0.6 |
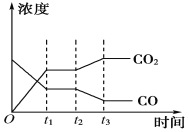
ДгЩЯБэПЩвдЭЦЖЯЃКДЫЗДгІЪЧ__________(ЬюЁАЮќЁБЛђЁАЗХЁБ)ШШЗДгІЁЃ
дк830 ЁцЯТЃЌШєПЊЪМЪБЯђКуШнУмБеШнЦїжаГфШыCOгыH2OОљЮЊ1 molЃЌдђДяЕНЦНКтКѓCOЕФзЊЛЏТЪЮЊ________ЁЃ
(2)ДЫСїГЬЕФЕкЂђВНЗДгІCO(g)ЃЋH2O(g) ![]() H2(g)ЃЋCO2(g)ЃЌдк830 ЁцЪБЃЌвдЯТБэЕФЮяжЪЕФСП(ЕЅЮЛЮЊmol)ЭЖШыКуШнЗДгІЦїЗЂЩњЩЯЪіЗДгІЃЌЦфжаЗДгІПЊЪМЪБЃЌЯђе§ЗДгІЗНЯђНјааЕФга________(ЬюЪЕбщБрКХ)ЁЃ
H2(g)ЃЋCO2(g)ЃЌдк830 ЁцЪБЃЌвдЯТБэЕФЮяжЪЕФСП(ЕЅЮЛЮЊmol)ЭЖШыКуШнЗДгІЦїЗЂЩњЩЯЪіЗДгІЃЌЦфжаЗДгІПЊЪМЪБЃЌЯђе§ЗДгІЗНЯђНјааЕФга________(ЬюЪЕбщБрКХ)ЁЃ
ЪЕбщБрКХ | n(CO) | n(H2O) | n(H2) | n(CO2) |
A | 1 | 5 | 2 | 3 |
B | 2 | 2 | 1 | 1 |
C | 0.5 | 2 | 1 | 1 |
(3)дквЛИіВЛДЋШШЕФЙЬЖЈШнЛ§ЕФШнЦїжаЃЌХаЖЯДЫСїГЬЕФЕкЂђВНЗДгІДяЕНЦНКтЕФБъжОЪЧ________(ЬюађКХ)ЁЃ
ЂйЬхЯЕЕФбЙЧПВЛдйЗЂЩњБфЛЏЁЁЂкЛьКЯЦјЬхЕФУмЖШВЛБфЁЁЂлЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПВЛБфЁЁЂмИїзщЗжЕФЮяжЪЕФСПХЈЖШВЛдйИФБфЁЁЂнЬхЯЕЕФЮТЖШВЛдйЗЂЩњБфЛЏЁЁЂоv(CO2е§)ЃНv(H2OФц)
(4)ЯТЭМБэЪОДЫСїГЬЕФЕкЂђВНЗДгІдкt1ЪБПЬДяЕНЦНКтЃЌдкt2ЪБПЬЗжБ№вђИФБфФГИіЬѕМўЖјЗЂЩњБфЛЏЕФЧщПіЃКЭМжаt2ЪБПЬЗЂЩњИФБфЕФЬѕМўПЩФмЪЧ________________________(аДГіСНжж)ЁЃ