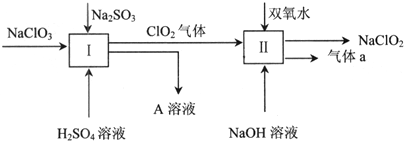
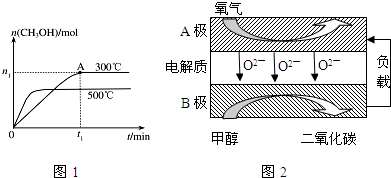
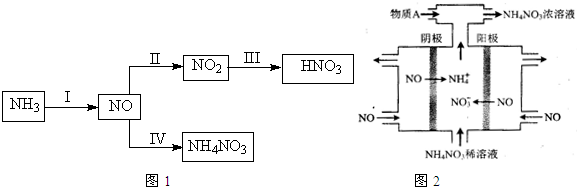
��Ŀ����
7������ʮ�ŷɴ����й������ۡ���ϵ�зɴ�֮һ�������й������Ҵ���̫���˵ķɴ�������ʮ�ŷɴ�����ɹ������칬һ��Ŀ������������������Ľ���Խӣ�����չ����ؿռ��ѧ���飮����ʮ�ŷɴ����ɳ�������F�Ľ������ػ����ңʮ����������������գ�����ƽ����dzɹ��������Ҫ���أ���ʵ�ϣ��ƽ����ķ�չ������һ�������Ĺ��̣���1��20����ǰ���ڻ�ҩ��������Ψһ�Ļ���ƽ������ڻ�ҩ��������ء���ǡ�ľ̿��ɣ��ڻ�ҩ��ը�Ļ�ѧ����ʽΪ��S+3C+2KNO3=K2S+N2��+3CO2����
��K2S�ĵ���ʽΪ
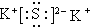 ��CO2�ĽṹʽΪO=C=O��
��CO2�ĽṹʽΪO=C=O������֪S����ˮ��Ӧ����������ǿ�ᣬ�����ӷ���ʽΪS+3Cl2+4H2O=SO42-+8H+6Cl-��
��2��20����60��������ʹ�õ���Һ���ƽ��������õ���������������������Һ���ȣ���ȼ�����£�N2H4����Һ��ȣ��£�N2H4������ˮ�Լ��ԣ���ԭ���백���ƣ�������Բ��簱ǿ��д��������ˮ�ʼ��Ե����ӷ���ʽ��N2H4+H2O?NH2NH3++OH-��
��3�����ϵĻ���ƽ���һ�㺬�е�Ԫ�أ���������������ḻ����һ������������к�ǿ�ı�ը�ԣ�86g�û����ﱬը�ֽ�����ɱ����N267.2L����һ�����嵥��H2��д���䱬ը�Ļ�ѧ����ʽ2HN3$\frac{\underline{\;��ը\;}}{\;}$3N2+H2��
���� ��1����K2S�����ӻ���������Ӻͼ������γ����Ӽ����ݴ�д������ʽ��C02��ֱ���η��ӣ�̼ԭ����2����ԭ�Ӷ��γ�˫�����ݴ�д���ṹʽ��
��S����ˮ��Ӧ����������ǿ�ᣬ��֪��Ϊ��������ᣬȻ����ƽ��
��2������NH3+H2O?NH4++OH-������������ˮ�ʼ��Ե����ӷ���ʽ��
��3���ȸ��������غ�ȷ��������������Ȼ�������ԭ�ӡ���ԭ�ӵ����ʵ��������ߵ����ʵ���֮�ȣ�ȷ��������ķ���ʽ�������д��ѧ����ʽ��
��� �⣺��1�����������ӻ���������Ӻͼ������γ����Ӽ����صĵ���ʽΪ��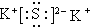 C02��ֱ���η��ӣ�̼ԭ����2����ԭ�Ӷ��γ�˫�����ṹʽΪO=C=O���ʴ�Ϊ��
C02��ֱ���η��ӣ�̼ԭ����2����ԭ�Ӷ��γ�˫�����ṹʽΪO=C=O���ʴ�Ϊ��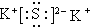 �� O=C=O��
�� O=C=O��
��S����ˮ��Ӧ��������������ᣬ��Ӧ�����ӷ���ʽΪ��3Cl2+S+4H2O=8H++SO42-+6Cl-��
�ʴ�Ϊ��3Cl2+S+4H2O=8H++SO42-+6Cl-��
��2��������ˮ�ķ�ӦΪ��NH3+H2O?NH4++OH-���£�N2H4������ˮ�Լ��ԣ���ԭ���백���ƣ����ӷ���ʽΪ��N2H4+H2O?NH2NH3++OH-��
�ʴ�Ϊ��N2H4+H2O?NH2NH3++OH-��
��3��86g�û����ﱬը�ֽ�����ɱ����N2 67.2L����һ�����嵥��H2��N2�����ʵ���Ϊ$\frac{67.2L}{22.4L/mol}$=3mol������Ϊ3mol��28g/mol=84g��������������Ϊ86g-84g=2g�����ʵ���Ϊ1mol����ԭ�ӡ���ԭ�ӵ����ʵ����ֱ�Ϊ6mol��2mol�����ߵ����ʵ���֮��Ϊ3��1������������ķ���ʽΪHN3����ѧ����ʽΪ��2HN3$\frac{\underline{\;��ը\;}}{\;}$3N2+H2��
�ʴ�Ϊ��2HN3$\frac{\underline{\;��ը\;}}{\;}$3N2+H2��
���� ���⿼���˺�����������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ��漰֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ��������Ϣ������⡢���������������֪ʶ��Ǩ��������������

| ���� | ���� | ���� | |
| A | ��ij��Һ�еμ�ϡNaOH��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ԭ��Һ����NH4+ |
| B | ��ij��ɫ��Һ�еμ���ˮ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ����I- |
| C | �ýྻ��˿պȡij��Һ������ɫ��Ӧ | ����ʻ�ɫ | ��Һ����K+ |
| D | ��ij����ͨ��Ʒ����Һ�� | Ʒ����Һ��ɫ | ������һ����SO2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 3��2��2��4 | B�� | 1��1��1��1 | C�� | 2��1��1��2 | D�� | 1��2��1��2 |
| A�� | һ�ȼ��飨CH3Cl�� | B�� | ����-CH3 �� | C�� | ̼�����ӣ�CH3-�� | D�� | ��ϩ��C2H4�� |