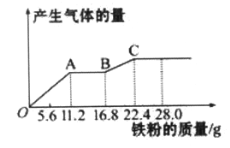
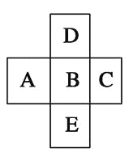
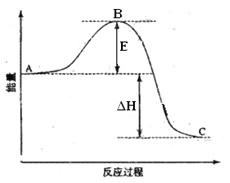
��Ŀ����
����Ŀ����֪1Lij����Һ�г�����0.2mol��L-1��Na+�⣬�����ܺ������������е�һ�ֻ��֣�
������ | K+��NH4+��Mg2+��Ba2+�� Fe3+ |
������ | Cl-��Br-��CO32-��HCO3-��SO42- |
�ֽ�������ʵ�����(ÿ��ʵ�������Լ�������)��

(1)������B��ȷ������Һ�к��е�������______________��
(2)�ɳ���D�ͳ���E�����жϴ���Һ��һ������______���ӣ��ݴ˿����ų���������________��
(3)�ɰ�ɫ����B��ȷ������Һ�к��е�������__________________��
(4)ijͬѧ��ͼ����Ϊ����Һһ�����������ӣ����жϵ�������_________________��
(5)�ۺϷ���������Һ��K+����СŨ��Ϊ____________________��
���𰸡�NH4+ CO32-��SO42- Mg2+��Ba2+��Fe3+ HCO3- ��ҺBͨ����������Һ��dz����ɫ����ҺB �μ������ữ��������Һ�����ְ�ɫ���� 0.1mol��L-1
��������
��1������ҺA����NaOH��Ӧ��������B����B�϶�ΪNH3�������Һ�к��е�������NH4+��n(NH4+)=n(NH3)=0.1mol����2������A�����ܽ���ϡ�����У���Һ��һ������CO32-��SO42-���ݴ˿����ų�Mg2+��Ba2+��Fe3+����3����Ӧ�����ӷ���ʽΪ��HCO3����OH����Ba2��=BaCO3����H2O��ȷ������Һ�к��е�������HCO3-��n(BaCO3)=n(HCO3-)=19.7g/197g/mol=0.1mol����4������Һһ�����������ӣ���Ϊ��ҺBͨ����������Һ��dz����ɫ����ҺB�μ������ữ��������Һ�����ְ�ɫ��������5�������ᱵ����Ϊ11.65g��n(BaSO4)=n(SO42-)=11.65g/233g/mol=0.05mol��̼��Ƶ�����Ϊ10.0g��n(CaCO3)=n(CO32--)=10.0g/100g/mol=0.1mol�������Ϸ�����֪��Na+���ʵ���Ũ��Ϊ0��2 mol��L��1��NH4+�����ʵ���Ũ��Ϊ0.1mol��L��1��CO32�����ʵ���Ũ��Ϊ0��1 mol��L��1��HCO3�����ʵ���Ũ��Ϊ0��1 mol��L��SO42�����ʵ���Ũ��Ϊ0��05 mol��L��1��Br-���ʵ���Ũ��Ϊ0 mol��L��1��Cl-���ʵ���Ũ����K+��Ũ��ȷ��������δ֪�����ݵ���غ㣬����Һ����Cl-��K+�����ʵ���Ũ��Ϊ0.1mol��L��1��������Cl-��K+�����ʵ���Ũ����СΪ0.1mol��L��1��