��Ŀ����
����Ŀ����1����״���£�1.92gij��������Ϊ672mL����������Ħ������Ϊ______��
��2��5.4g H2O������ԭ����Ŀ��_____L����״��������������ԭ����Ŀ��ȣ�
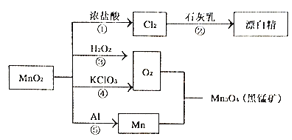
��3��A��B��C��D��Ϊ��ѧ��ѧ�ij��������Ҿ�����ͬһ��Ԫ�أ�����֮���ת����ϵ������ʾ����Ӧ���������������Ѿ���ȥ���� A![]() B
B![]() C
C![]() D
D
a.��A��һ�ֽ�����C�ǵ���ɫ���壬д��C��һ����;________��
b.��AΪ����ɫ���嵥�ʣ�ʵ���ҳ����ڼ���B���Լ���__________��
c.��A�ǻ����C�Ǻ���ɫ���壬��D�Ļ�ѧʽΪ_______��
���𰸡�64g/mol 4.48 L ������ Ʒ����Һ HNO3
��������
��1������£�672mL��������ʵ���Ϊ��![]() = 0.03mol�������Ħ������Ϊ��M=1.92g��0.03mol=64g/mol����Ϊ64g/mol��
= 0.03mol�������Ħ������Ϊ��M=1.92g��0.03mol=64g/mol����Ϊ64g/mol��
��2��H2O����ԭ����Ŀ��NH3����ԭ����Ŀ��ȣ������ǵ���ԭ�ӵ����ʵ������ ����5.4gH2O�к���ԭ�ӵ����ʵ���n(H)= ![]() =0.6mol�������VL��NH3����ԭ�ӵ����ʵ���ҲΪ0.6mol����
=0.6mol�������VL��NH3����ԭ�ӵ����ʵ���ҲΪ0.6mol����![]() =0.6mol����V=4.48L����Ϊ4.48L��
=0.6mol����V=4.48L����Ϊ4.48L��
��3��a����A��һ�ֽ�����C�ǵ���ɫ���壬��CӦΪ�������ƣ����Ƴ�AΪ�ƣ�B�������ƣ�C�ǹ������ƣ�D���������ƣ��������Ƶ�һ����;Ϊ�������DZˮͧ�й���������Ϊ��������
b����������AΪ����ɫ���嵥�ʣ�����Ƴ�A������ת����ϵ֪��B�Ƕ�������C����������D�����ᣬ�������������Լ�����Ʒ����Һ����ΪƷ����Һ��
c����A�ǻ����C�Ǻ���ɫ���壬��CӦΪNO2����ô����ת����ϵ֪��BΪNO��DΪHNO3��������AӦΪNH3����ΪHNO3��

 ����5��2���ϵ�д�
����5��2���ϵ�д�����Ŀ�������£����ʵ���֮��Ϊ2��1��SO2��O2�Ļ���������ݻ�Ϊ2 L�ĺ����ܱ������з�����Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)(����ӦΪ���ȷ�Ӧ)��n(SO2)��ʱ��仯��ϵ���±���
2SO3(g)(����ӦΪ���ȷ�Ӧ)��n(SO2)��ʱ��仯��ϵ���±���
ʱ��/min | 0 | 1 | 2 | 3 | 4 | 5 |
n(SO2)/mol | 0.20 | 0.16 | 0.13 | 0.11 | 0.08 | 0.08 |
����˵����ȷ����( )
A. ��������������ܶȲ���ʱ���÷�Ӧ�ﵽƽ��״̬
B. �÷�Ӧ���е���3����ʱ���淴Ӧ����С������Ӧ����
C. �ӷ�Ӧ��ʼ���ﵽƽ�⣬��SO3��ʾ��ƽ����Ӧ����Ϊ0.01 mol/(L��min)
D. �����ڴﵽƽ��״̬ʱ��ѹǿ����ʼʱ��ѹǿ֮��Ϊ5��4