��Ŀ����
����Ŀ���ں��¡����������·������з�Ӧ��2X2O5 (g) ![]() 4XO2(g) +O2(g) ��H>0��T�¶��µIJ���ʵ������Ϊ��
4XO2(g) +O2(g) ��H>0��T�¶��µIJ���ʵ������Ϊ��
t/s | 0 | 50 | 100 | 150 |
c(X2O5) mol/L | 4.00 | 2.50 | 2.00 | 2.00 |
����˵���������
A. T�¶��µ�ƽ����ΪK=64 (mol/L)3��100sʱת����Ϊ50%
B. 50s �� X2O5�ֽ�����Ϊ 0.03 mol/ (Ls)
C. T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1>T2����Kl>K2
D. ��ֻ�����ݸ�Ϊ��ѹ���������������䣬��ƽ��ʱX2O5��ת���ʺ�ƽ�ⳣ��������
���𰸡�D
��������
A���ɱ������ݿ�֪��100sʱ����ƽ��״̬��ƽ��ʱc(X2O5)=2mol/L����
2X2O5(g)�T4XO2(g)+O2(g)
��ʼŨ��(mol/L)��4 0 0
�仯Ũ��(mol/L)��2 4 1
ƽ��Ũ��(mol/L)��2 4 1
T�¶���ƽ�ⳣ��K=![]() =
=![]() =64(mol/L)3��100sʱת����Ϊ
=64(mol/L)3��100sʱת����Ϊ![]() ��100%=50%����A��ȷ��B.50s��X2O5Ũ�ȱ仯��Ϊ(4-2.5)mol/L=1.5mol/L��50s��X2O5�ֽ�����=
��100%=50%����A��ȷ��B.50s��X2O5Ũ�ȱ仯��Ϊ(4-2.5)mol/L=1.5mol/L��50s��X2O5�ֽ�����=![]() =0.03mol/(L��s)����B��ȷ��C��T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2�����¶�T1��T2������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ��������K1��K2����C��ȷ��D�����������£��淴Ӧ����������ѹǿ������ֻ�����ݸ�Ϊ��ѹ���������������䣬��ЧΪ��ԭƽ������ϼ�Сѹǿ��ƽ�������ƶ���ƽ��ʱX2O5��ת��������ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣬��D����ѡD��
=0.03mol/(L��s)����B��ȷ��C��T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2�����¶�T1��T2������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ�ⳣ��������K1��K2����C��ȷ��D�����������£��淴Ӧ����������ѹǿ������ֻ�����ݸ�Ϊ��ѹ���������������䣬��ЧΪ��ԭƽ������ϼ�Сѹǿ��ƽ�������ƶ���ƽ��ʱX2O5��ת��������ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣬��D����ѡD��

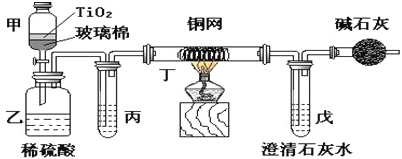
����Ŀ������ʵ��װ�ã�����װ������ȥ��������������ǣ� ��
|
|
|
|
A�����հ����������� | B����SO2��Ba(NO3)2��Ӧ���BaSO3���� | C�������屽�ͱ��Ļ���� | D. ��֤HCl���ܽ��� |
A. A B. B C. C D. D