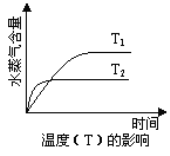��Ŀ����
����Ŀ�����������[(NH4)2Fe(SO4)2��6H2O�������������ױ������������Ƿ�����ѧ����Ҫ���Լ��������ڴ������������������������500��ʱ��������������ȫ�ֽ⣬�ش��������⣺
(1)��������笠�������������ȫ�ֽⷢ����������ԭ��Ӧ��������������FeO��Fe2O3��������������NH3��SO3��H2O��N2��_________��
(2)Ϊ����ֽ����ijɷ֣��������ʵ��װ�ý���ʵ�飬����A�е�������������ֽ���ȫ��
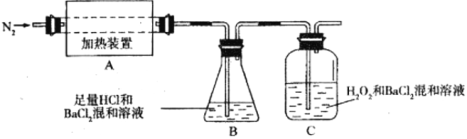
��Ϊ��֤A�в������Ƿ���FeO����Ҫѡ�õ��Լ���_________��
A.KSCN��Һ B.ϡ���� C.Ũ���� D.KMnO4��Һ
��װ��B��BaCl2��Һ��������Ϊ�˼���ֽ�������Ƿ���_________�������ɣ������и����壬�۲쵽������Ϊ___________________��
����A�зֽ������N2�����������ֻ��Fe2O3��Fe2O3�����ʵ���Ϊbmol��C�г������ʵ���Ϊamol����b_________a(����ڡ�����С�ڡ����ڡ�)
��ʵ���У��۲쵽C���а�ɫ�������ɣ���C�з�����Ӧ�����ӷ���ʽΪ________________��
(3)Ϊ�ⶨij������Ʒ��(NH4)2Fe(SO4)2��6H2O(M=392g/mol)�ĺ�����ijʵ��С����������ʵ�飺MnO4��+Fe2++H+=Mn2++Fe3++H2O(����ʽδ��ƽ)
�ⶨ���裺
����һ��ȷ����20.00g��������茶��壬���Ƴ�100mL��Һ��
�������ȡ������Һ25.00mL����ƿ�У���ϡH2SO4�ữ����0.1000mol��L��1KMnO4��Һ�ζ����յ㣬�ظ����Σ�ƽ������KMnO4��Һ16.00mL��
�ٲ�����ﵽ�ζ��յ�ı�־Ϊ________________________��
�ڲ�Ʒ��(NH4)2Fe(SO4)2��6H2O����������Ϊ____________________��
���𰸡�SO2 BD SO3 ��Һ����� С�� SO2+ H2O2+ Ba2+ = BaSO4��+2H+ �������һ�θ������ʱ����Һ���Ϻ�ɫ���Ұ����֮�ڲ���ɫ 62.72 %
��������
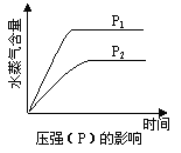
��1����Ԫ�غ���Ԫ�ػ��ϼ����ߣ�������������ԭ��Ӧ�л��ϼ��������н������ϼ۽��͵�Ӧ�����ݴ˷���������
��2����FeOΪ���������������ˮ���������ᣬ���ױ����������ݴ��ص㣬�ɿ��Ǽ�������KMnO4��Һ����������Fe3+��KMnO4��Һ��������ɫ������Ҫ����ָʾ����Ũ�����ױ�����������������ѡ�õ��Լ�Ϊϡ������KMnO4��Һ����ѡBC��
��BaC12����SO3��Ӧ���ɰ�ɫ������
����A�зֽ������N2�����������ֻ��Fe2O3��Fe2O3�����ʵ���Ϊbmol��C�г������ʵ���Ϊamol�����ݻ��ϼ�������ȷ���������
����˫��ˮ���������ԣ��ɽ�SO2������SO42-��SO42-����Ba2+��ϳɳ���BaSO4���ݴ˷�������
��3����ȡ������Һ25.00 mL����ƿ�У���ϡH2SO4�ữ����0.1000molL-1 KMnO4��Һ�ζ������������һ�θ��������Һ���Ϻ�ɫ�Ұ���Ӳ��䣬˵����Ӧ���յ㣻
�ڽ�ϻ�ѧ��Ӧ������ϵ���㣬MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
��1����Ԫ�غ���Ԫ�ػ��ϼ�������������������ԭ��Ӧ�л��ϼ��������н������ϼ۽��͵�Ӧ��������������������л�������SO2��
�ʴ�Ϊ��SO2��
��2����FeOΪ���������������ˮ���������ᣬ���ױ����������ݴ��ص㣬�ɿ��Ǽ�������KMnO4��Һ����������Fe3+��KMnO4��Һ��������ɫ������Ҫ����ָʾ����Ũ�����ױ�����������������ѡ�õ��Լ�Ϊϡ������KMnO4��Һ��B��D��ȷ��
�ʴ�Ϊ��BD��
��װ��B��BaC12��Һ��������Ϊ�˼���ֽ�������Ƿ���SO3�������ɣ������и����壬���������ᱵ��ɫ�������仯ѧ����ʽΪ��BaC12+SO3+H2O = BaSO4��+2HC1����۲쵽�Ĺ���Ϊ��Һ����ǣ�
�ʴ�Ϊ��SO3����Һ����ǣ�
����A�зֽ������N2�����������ֻ��Fe2O3��Fe2O3�����ʵ���Ϊb mol��C�г������ʵ���Ϊa mol�����ݻ��ϼ�������������͵Ļ��ϼ�(2a)���������ߵĻ��ϼ�(2b)�͵����ߵĻ��ϼ�֮�ͣ���bС��a��
�ʴ�Ϊ��С�ڣ�
��C���а�ɫ��������,����ΪSO2��˫��ˮ������SO42��SO42����Ba2+��ϳɳ���BaSO4����C�з����ķ�ӦΪSO2+ H2O2+ Ba2+ = BaSO4��+2H+��
�ʴ�Ϊ��SO2+ H2O2+ Ba2+ = BaSO4��+2H+��
(3)��ȡ������Һ25.00 mL����ƿ������ϡH2SO4�ữ����0.1000 molL1KMnO4��Һ�ζ����յ����μ����һ��KMnO4��Һʱ����Һ���Ϻ�ɫ�Ұ���Ӳ���ɫ��
�ʴ�Ϊ���μ����һ��KMnO4��Һʱ����Һ����ɫ�Ұ���Ӳ���ɫ��
�� MnO4+5Fe2++8H+=Mn2++5Fe3++4H2O
1 5
0.01600 L��0.1000 molL1 n
n=5��0.01600 L��0.1000 mol L-1=0.008 mol��
100 mL��Һ�к������������ʵ��� = 0.008 mol��![]() = 0.032 mol��
= 0.032 mol��
��Ʒ��(NH4)2Fe(SO4)2��6H2O���������� = ![]() ��100% = 62.72%��
��100% = 62.72%��
�ʴ�Ϊ��62.72%��

����Ŀ������ʵ���ʵ�������ʵ�������ʵ����۶���ȷ����
ѡ�� | ʵ����� | ʵ������ | ʵ����� |
A | �����£��������ϵμ�Ũ���� | ���������� | ����Ũ�����Ӧ |
B | ����������ͨ����ɫʯ����Һ | �ȱ�����ɫ | �������������������������Ư���� |
C | ���Ȼ�������Һ�еμ�����KBrϡ��Һ | ��ɫ���DZ�Ϊ����ɫ���� | Ksp(AgCl)>Ksp (AgBr) |
D | NaAlO2��Һ�еμ� NaHCO3��Һ | ������ɫ���� | N aAlO2��Na HCO3������ٽ���ˮ�ⷴӦ |
A. A B. B C. C D. D