��Ŀ����
����Ҫ��������и�С��
��1����ʵ�����ü��ȹ�������ķ����Ʊ������Ļ�ѧ��Ӧ����ʽ�� ��
��Ϊ�˵õ������NH3����________��������������ţ�
| A����ʯ�� | B��ŨH2SO4 | C����ˮCaCl2 | D��P2O5 |
����X+W��Y+V����֪X��Y�ֱ��Ƕ�����ͬ��������Ԫ���γɵĵ��ʣ� W��V�ǻ�����
��W��ˮ��V����ɫ��ӦΪ��ɫ�����ӷ���ʽ ��
��V��ˮ����ѧ����ʽΪ ��
(7��)
��1����2NH4Cl + Ca(OH)2 2NH3�� + CaCl2 + 2H2O��2�֣�
2NH3�� + CaCl2 + 2H2O��2�֣�
��A��1�֣�
(2)��2Na +2 H2O ="2Na+" +2OH- + H2����2�֣�
��2H2S+O2 ==2S+2H2O��2�֣�
���������������1����ʵ�����ü��ȹ�������ķ����Ʊ������Ļ�ѧ��Ӧ����ʽ��2NH4Cl + Ca(OH)2 2NH3�� + CaCl2 + 2H2O��Ϊ�˵õ������NH3����A����ʯ�������������2����W��ˮ��V����ɫ��ӦΪ��ɫ��˵��V�к�����Ԫ�أ����ӷ���ʽ2Na +2 H2O ="2Na+" +2OH- + H2����V��ˮ�� ��ѧ����ʽΪ2H2S+O2 ==2S+2H2O��
2NH3�� + CaCl2 + 2H2O��Ϊ�˵õ������NH3����A����ʯ�������������2����W��ˮ��V����ɫ��ӦΪ��ɫ��˵��V�к�����Ԫ�أ����ӷ���ʽ2Na +2 H2O ="2Na+" +2OH- + H2����V��ˮ�� ��ѧ����ʽΪ2H2S+O2 ==2S+2H2O��
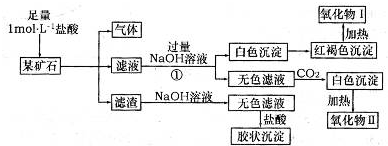
���㣺���⿼�鰱�����Ʊ�������ˮ����ķ�Ӧ����ʽ����д��

����Һ(������)X������(��ͨ��)��һ������Y��Һ�У������������������X���ʵ����Ĺ�ϵ����ͼ������ͼ�������һ��������(����)��
| | A | B | C | D |
| X | H2S | HCl | NH3 | NH3��H2O |
| Y | Na2SO3 | NaAlO2 | AlCl3 | AgNO3 |
�����ڵ�����Ԫ��A��B��C��D��E,ԭ��������������A��B��C����Ԫ�ص��Ӳ���֮����5��A��B��Ԫ��ԭ������������֮�͵���CԪ��ԭ������������;BԪ��ԭ��������Ӳ��ϵĵ����������ĵ��Ӳ�����2��,A��D�����γ�ԭ�Ӹ����ȷֱ�Ϊ1��1��2��1������Һ̬������;E�������ھ���ˮ�ʡ�
��ش�:
(1)д��D��Ԫ�����ڱ��е�λ���� ,
E��ԭ�ӽṹʾ��ͼ��������������������
���п�����֤C��D��Ԫ��ԭ�ӵõ�������ǿ����ʵ����ʵ����������(��д���)��
A.�Ƚ�������Ԫ�ص���̬�⻯��ķе�
B.�Ƚ�ֻ��������Ԫ�����γɵĻ������еĻ��ϼ�
C.�Ƚ�������Ԫ�ص���̬�⻯����ȶ���
D.�Ƚ�������Ԫ�صĵ������������ϵ�����
(2)��A��B����Ԫ����ɵ���Ļ�����,д�������ʽ����������
(3)����A��B��C��D����Ԫ����ɵļס������ֻ�����,���ȿ��������ᷴӦ�ֿ�����NaOH��Һ��Ӧ,��Ϊ����,�仯ѧʽΪ��������,��Ϊ��Ȼ�߷��ӻ������ˮ�����,����ͬ����������Է���������С��,��ṹ��ʽΪ������������������������
(4)��̬��������ҽѧ������Ҫ����;,������Fe3O4�Ǵ������е���Ҫ����,���Ʊ����̿ɼ�ʾ����:
�ٽ�������CA3ͨ������ʵ�����FeSO4��Fe2(SO4)3�Ļ����Һ��,�������ּ�,д���÷�Ӧ���̵��ܵ����ӷ���ʽ�� ��
��������Ӧ���ɵ����ּ��������,�õ�Fe3O4��
(5)��֪�±�����:
| ���� | Fe(OH)2 | Fe(OH)3 |
| Ksp/25 �� | 2.0��10-16 | 4.0��10-36 |
��ʹ���Һ��FeSO4��Fe2(SO4)3��Ũ�Ⱦ�Ϊ2.0 mol��L-1,����Һ��c(OH-)���ô�����������mol��L-1��
A��B��C��D��ԭ��������������Ķ���������Ԫ�أ�A��C��Ԫ�����ڱ��е����λ����ͼ��AԪ��������������ϵĵ�����֮��Ϊ3��BΪ�ؿ��к������Ľ���Ԫ�ء�
| A | |
| | C |
��1��Dԭ�ӽṹʾ��ͼΪ_____________��
��2����C�ĵͼ�̬�������ͨ�뵽D���ʵ�ˮ��Һ��ʹ֮��ɫ�������˼�________�ԣ�д���÷�Ӧ�����ӷ���ʽ_____________________��
��3��A������������Ӧ��ˮ�������ң��ֽ�����Cu���뵽100 mL 8.0 mol/L�ҵ�Ũ��Һ�У���ַ�Ӧ�����ռ���6.72L����״�������壬�������ijɷ���_______________����ԭ��ʧ������Ϊ_________________��
��4��������������B���ʷֱ���뵽�������Ũ�ȵ������NaOH��Һ�У���ַ�Ӧ��������������Ϊ____________________��������Ӧ�����õ���Һ��ϣ������ɰ�ɫ������������Ӧ�����ӷ���ʽΪ___________________________________________________��B���ʱ��������Ĥ����NaOH��Һ��ȥ��д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________________________________��
W��X��Y��Z���ֶ���������Ԫ�ص�ԭ��������������W��Xԭ�ӵ�����������֮��Ϊ4:3��Zԭ�ӱ�Xԭ�ӵĺ����������4������˵����ȷ����
| A��W��Y��Z�ĵ縺�Դ�С˳��һ����Z>Y>W |
| B��W��X��Y��Z��ԭ�Ӱ뾶��С˳�������W>X>Y>Z |
| C��Y��Z�γɵķ��ӵĿռ乹�Ϳ������������� |
| D��WY2�����Цļ���м�����Ŀ֮����2:1 |