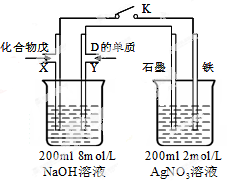
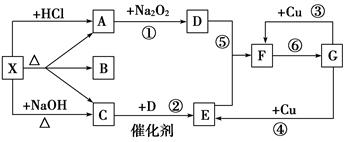
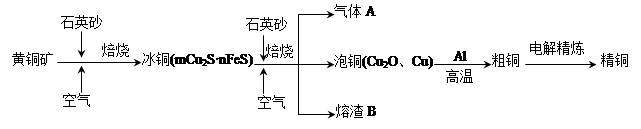��Ŀ����
��ѧ������ij��Ӧ�Ļ�ѧ����ʽΪa��b�D��c��d��H2O(δ��ƽ����Ӧ��������ȥ)��
��ش��������⣺
(1)��a������b��ϡ����(����)����a������c��Һ�С���a��b��Ӧ�����ӷ���ʽΪ
___________________________��
(2)��c��dΪ���壬�Ҷ���ʹ����ʯ��ˮ����ǣ��˻������ͨ����ˮ�У���ɫ��ȥ��д������ɫ�����з�����Ӧ�����ӷ���ʽ��___________________________��
(3)��c����ɫ�д̼�����ζ�����壬��ˮ��Һ�������ԣ��ڱ�״�������ſ������ռ�c���壬��ƽ��Ħ������Ϊ20 g��mol��1�Ļ�����������Ȫʵ�顣�������ʲ���ɢ��ʵ����ɺ���ƿ��������Һ�����ʵ���Ũ��Ϊ________mol��L��1(С�������2λ��Ч����)��
(4)��a���������ЧӦ����Ҫ���壬c��d��Ϊ���Σ��μӷ�Ӧ��a��b�����ʵ���֮��Ϊ4:5����������Ӧ�����ӷ���ʽΪ____________________________��
(1)Fe��4H����NO3��=Fe3����NO����2H2O
(2)SO2��2H2O��Br2=4H����SO42����2Br��
(3)0.045
(4)4CO2��5OH��=3HCO3����CO42����H2O
����

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д��±��У��Գ���I�������ȷ�Լ������Ƿ���������ϵ���ж϶���ȷ����
| ѡ�� | ����I | ������ | �ж� |
| A | KSCN��Һ�ɼ���Fe3+ | ����Һ�Ⱥ����������ˮ�ͼ���KSCN��Һ���ΪѪ��ɫ��֤������Һ��Fe3+ | I�ԣ���ԣ��� |
| B | SO2���л�ԭ�� | SO2ͨ��Ba(NO3)2��Һ�пɼ���ɫ�������� | I�ԣ���ԣ��� |
| C | NO2�����������ʺ���ɫ | ��ˮ�ɼ���NO2�������� | I�ԣ�������� |
| D | ��Ӧ�������ͬ�ɵ��²��ﲻͬ | Na��O2��Ӧ��������Na2O��Ҳ��������Na2O2 | I�ԣ���ԣ��� |
����Ҫ��������и�С��
��1����ʵ�����ü��ȹ�������ķ����Ʊ������Ļ�ѧ��Ӧ����ʽ�� ��
��Ϊ�˵õ������NH3����________��������������ţ�
| A����ʯ�� | B��ŨH2SO4 | C����ˮCaCl2 | D��P2O5 |
����X+W��Y+V����֪X��Y�ֱ��Ƕ�����ͬ��������Ԫ���γɵĵ��ʣ� W��V�ǻ�����
��W��ˮ��V����ɫ��ӦΪ��ɫ�����ӷ���ʽ ��
��V��ˮ����ѧ����ʽΪ ��