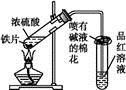
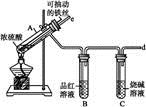
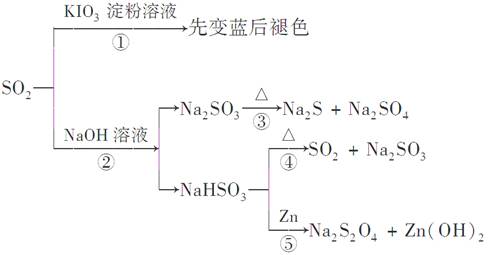
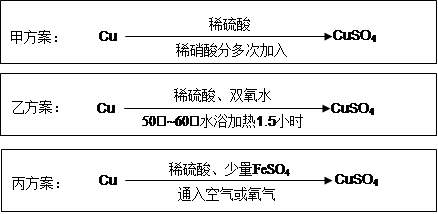��Ŀ����
��ˮ����Ҫ����Na+��K+��Mg2+��Ca2+��Cl����Br����SO42-��HCO3-��CO32-�����ӣ���������ʱ�ŷŵ��������ú�ˮ�����乤����������ͼ��ʾ������˵���������


| A����ˮpHԼΪ8��ԭ����Ҫ����Ȼ��ˮ��CO32-��HCO3- |
B���������з����ķ�Ӧ��SO2+H2O H2SO3 H2SO3 |
| C��������Ҫ��������HSO3-��SO32-��H2SO3����ΪSO42- |
| D����ϡ�͡��ŷš����ķ�ˮ�У�SO42-Ũ���뺣ˮ��ͬ |
D
��ˮ����Ҫ����Na+��K+��Mg2+��Cl-��SO42-��Br-��CO32-��HCO3-�����ӣ�����Щ�������ܷ���ˮ�����CO32-��HCO3-���ӣ�CO32-��HCO3-���ܷ���ˮ�⣬���ּ��ԣ�A��ȷ���������������ԣ�����SO42-��HSO3-��H2SO3������Ϊ���ᣬB��ȷ��������ĺ�ˮ��Ҫ����Ȼˮ��ϣ����ͺ�ˮ�е��ᣬ���ܴﵽ�ŷţ�C��ȷ������ϡ���뺣ˮ��ϣ�SO42-Ũ�ȱȺ�ˮҪС��D������ѡD��

��ϰ��ϵ�д�
�����Ŀ
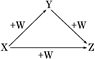

 N2����6NH3����3SO2����SO3����7H2O������Ӧ��Ļ��������ͨ�����ڱ�ˮԡ��U�ܣ���ͨ��������BaCl2��Һ�����Ϊ
N2����6NH3����3SO2����SO3����7H2O������Ӧ��Ļ��������ͨ�����ڱ�ˮԡ��U�ܣ���ͨ��������BaCl2��Һ�����Ϊ