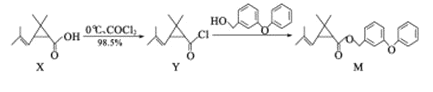
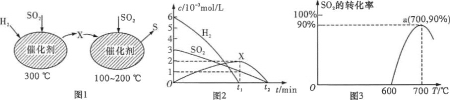
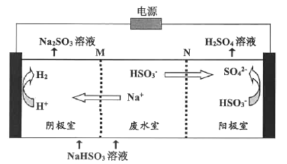
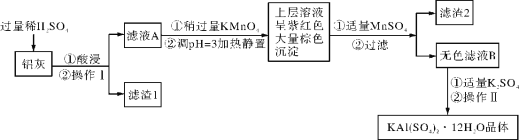
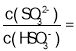��Ŀ����
����Ŀ��(1)��������һ�ַdz����õĽ�����ͨ������________�С�����Ͷ������ͭ��Һ�У�������Ӧ�����ӷ���ʽΪ_____________��_________________
(2)Na2O2����Ϊ��������еĹ��������乩��ʱ��Ӧ�Ļ�ѧ����ʽ�У�__________��_____
(3)��һ����Һ�����ܺ���Al3����Fe3����K����Mg2����Cu2���������е�һ�ֻ��֡��ּ���Na2O2��ĩ����ɫ��ζ������ų���ͬʱ������ɫ������������Һ�е�ˮ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ����ͼ����ʾ�����ƶϣ�
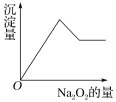
��ԭ��Һ��һ�����е�������__________________��
��һ�������е�������_________________��
�ۿ��ܺ���___________��Ϊ�˽�һ��ȷ�����ܺ��и����ӣ���������ɫ��Ӧ��ʵ�飬����ɫ�ܲ����۲쵽�Ļ������ɫΪ_______ɫ��
���𰸡�ú�� 2Na��2H2O=2Na����2OH����H2�� Cu2++2OH-=Cu(OH)2�� 2Na2O2��2CO2=2Na2CO3��O2 2Na2O2��2H2O=4NaOH��O2 �� Mg2����Al3�� Fe3����Cu2�� K�� ��
��������
��1������һ�ַdz����õĽ�����ͨ��������ú���У�����Ͷ������ͭ��Һ�У�������ˮ��Ӧ�����������ƺ��������̶��������ƻ������ͭ��Ӧ����������ͭ��ˮ��
�ʴ�Ϊ��ú�ͣ� 2Na��2H2O=2Na����2OH����H2����Cu2++2OH-=Cu(OH)2����
��2��������������������Ϊ��Ͷ�����̼��ˮ���ܷ�Ӧ����������
�ʴ�Ϊ��2Na2O2��2CO2=2Na2CO3��O2��2Na2O2��2H2O=4NaOH��O2 ����
��3������Na2O2��ĩ������������ˮ��Ӧ��2Na2O2+2H2O=4NaOH+O2������ͬʱ������ɫ�������ʿ��ƶϲ����������Ӻ�ͭ���ӣ�����������Ĺ�������ʱ�������ɰ�ɫ����������������ܽ⣬�ƶ�һ����Mg2+��Al3+�����ܺ���K+��K+�ļ��飺�ýྻ�IJ�˿պȡ����Һ���ھƾ��������գ�����ɫ�ܲ����۲�������ɫ���������ɫ��˵������K+��
�ʴ�Ϊ��Al3+��Mg2+��Fe3+��Cu2+��K+���ϡ�