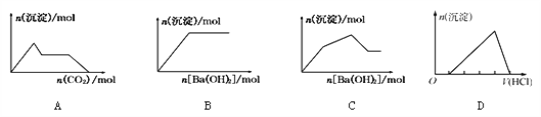
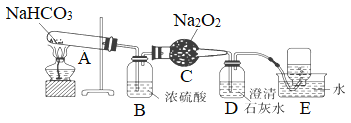
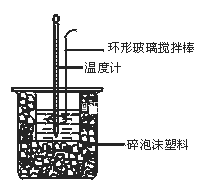
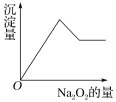
��Ŀ����
����Ŀ��д�����л�ѧ����ʽ�����ӷ���ʽ��
��1������ˮ��Ӧ�����ӷ���ʽ��___��
��2����������ͭ��Һ��Ӧ�����ӷ���ʽ��___��
��3�����������������̼��Ӧ�Ļ�ѧ����ʽ��___��
��4�������������������Ӧ�Ļ�ѧ����ʽ��___��
��5��þ�ڵ����е�ȼþ���Ļ�ѧ����ʽ��___��
��6��þ�ڶ�����̼�е�ȼþ���Ļ�ѧ����ʽ��___��
��7����������������Һ�ķ�Ӧ�����ӷ���ʽ��___��
��8��������������������Һ��Ӧ�����ӷ���ʽ��___��
��9����������������������Һ��Ӧ�����ӷ���ʽ��___��
��10������������������Ӧ�Ļ�ѧ����ʽ��___��
��11��ͭ���Ȼ�����Һ��Ӧ�����ӷ���ʽ��___��
���𰸡�2Na+2H2O=2Na++2OH�D+H2�� 2Na+Cu2++2H2O==Cu(OH)2��+H2��+2Na+ 2Na2O2+2CO2==2Na2CO3+O2 Na2O2+SO2==Na2SO4 3Mg+N2![]() Mg3N2 2Mg+CO2
Mg3N2 2Mg+CO2![]() 2MgO+C 2Al+2OH�C+2H2O=2AlO2�C+3H2�� Al2O3+2OH-=2AlO2-+H2O Al(OH)3+OH-=AlO2-+2H2O 8Al+3Fe3O4
2MgO+C 2Al+2OH�C+2H2O=2AlO2�C+3H2�� Al2O3+2OH-=2AlO2-+H2O Al(OH)3+OH-=AlO2-+2H2O 8Al+3Fe3O4![]() 4Al2O3+9Fe 2Fe3++Cu��2Fe2++ Cu2+
4Al2O3+9Fe 2Fe3++Cu��2Fe2++ Cu2+
��������
��1������ˮ��Ӧ�����ӷ���ʽ��2Na+2H2O=2Na++2OH-+H2����
��2����������ͭ��Һ��Ӧ�����ӷ���ʽ��2Na+Cu2++2H2O==Cu(OH)2��+H2��+2Na+��
��3�����������������̼��Ӧ�Ļ�ѧ����ʽ��2Na2O2+2CO2==2Na2CO3+O2��
��4�������������������Ӧ�Ļ�ѧ����ʽ��Na2O2+SO2==Na2SO4��
��5��þ�ڵ�����ȼ�յĻ�ѧ����ʽ��3Mg+N2![]() Mg3N2��
Mg3N2��
��6��þ�ڶ�����̼��ȼ�յĻ�ѧ����ʽ��2Mg+CO2![]() 2MgO+C��
2MgO+C��
��7����������������Һ�ķ�Ӧ�����ӷ���ʽ��2Al+2OH�C+2H2O=2AlO2�C+3H2����
��8��������������������Һ��Ӧ�����ӷ���ʽ��Al2O3+2OH-=2AlO2-+H2O��
��9����������������������Һ��Ӧ�����ӷ���ʽ��Al(OH)3+OH-=AlO2-+2H2O��
��10������������������Ӧ�Ļ�ѧ����ʽ��8Al+3Fe3O4![]() 4Al2O3+9Fe��
4Al2O3+9Fe��
��11��ͭ���Ȼ�����Һ��Ӧ�����ӷ���ʽ��2Fe3++Cu��2Fe2++ Cu2+��

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�