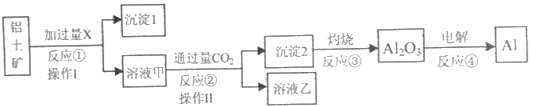
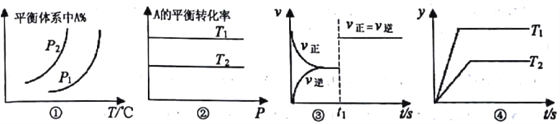
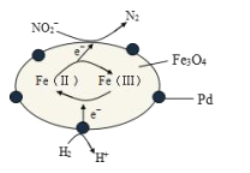
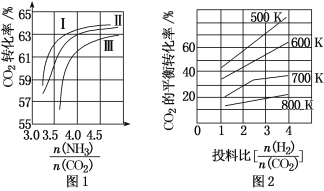
��Ŀ����
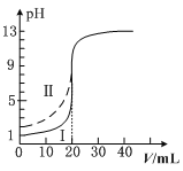
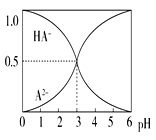
����Ŀ�������£�0.1 mol��L��1��ij��Ԫ��H2A��Һ�У����ܴ��ڵĺ�A����(H2A��HA����A2��)�����ʵ���������pH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����(����)
A. H2A�ĵ��뷽��ʽ��H2A![]() H����HA��
H����HA��
B. pH��5ʱ,��NaHA��Na2A�Ļ����Һ��:c(HA��):c(A2��)��1:100
C. �����ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������ϣ� ����Ũ�ȴ�С��ϵΪ��c(Na��)>c(HA��)>c(A2��)
D. Na2A��Һ�ش���c(OH��)��c(H��)��c(HA��)�� 2c(H2A)��������Ũ�Ⱦ�����0
���𰸡�B
�����������������A����Ԫ��H2A��Һ�в���������ӣ�˵����һ����ȫ���룬��Ԫ��H2A�ĵ��뷽��ʽΪ��H2A=H++HA-��HA-H++A2-����A����B����ͼ�������pH=3ʱ��c(HA-)��c(A2-)��ͬ��pH=5ʱ��c(HA-)��c(A2-)=1��100����B��ȷ��C�������ʵ���Ũ�ȵ�NaHA��Na2A��Һ�������Ϻ����Ƚ�c(HA-)��c(A2-)�Ĵ�С����C����D����Ԫ��H2A�ĵ��뷽��ʽΪ��H2A=H++HA-��HA-H++A2-��Na2A��Һ�в�����H2A���ӣ�c(H2A)=0����D���ʴ�ΪB��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ