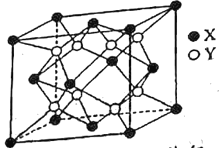
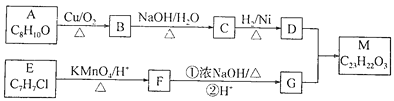
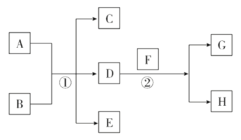
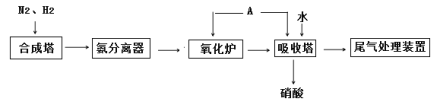
��Ŀ����
����Ŀ�������£���0.100mol��L1��NaOH��Һ�ֱ�ζ���Ϊ20.00mL0.100mol��L1��HCl��Һ�ʹ�����Һ���ζ�������ͼ��ʾ������˵����ȷ����
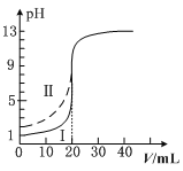
A.���ʾ���ǵζ����������
B.pH=7ʱ���ζ��������ĵ�V��NaOH����20.00mL
C.V��NaOH����20.00mLʱ��������Һ��c��Cl������c��CH3COO����
D.V��NaOH����10.00mLʱ��������c��Na+����c��CH3COO������c��H+����c��OH����
���𰸡�C
��������
A. 0.100mol��L-1��HCl��Һ�ʹ�����Һ�������������ᣬ���ڵ���ƽ�⣬�������pH��С�Ģ��ʾ���ǵζ���������ߣ�A�����
B. ����������������ǡ����ȫ��Ӧʱ���γɴ�������Һ��������ˮ��ʹ��ҺpH>7������pH =7ʱ���ζ��������ĵ�V(NaOH)<20.00mL��B�����
C. V(NaOH)= 20.00mLʱ�����ǡ����ȫ��Ӧ����ΪCH3COO-ˮ������ģ�����������Һ��c(Cl-����c(CH3COO-����C����ȷ��
D. V(NaOH)=10.00mLʱ�����ɵĴ�������ʣ�����Ũ����ȣ����ڴ���ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶ȣ�������Һ��c(CH3COO-)��c(Na+)��c(H+)��c(OH-)��D�����
��ѡC��