��Ŀ����
����Ŀ���������(K2FeO4)��һ�ּ�������������������һ������Ͷ��ˮ���������������������£�
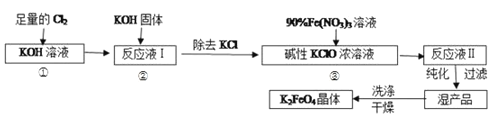
��֪����2KOH+Cl2=KCl+KClO+H2O(�������¶Ƚϵ�)
��6KOH+3Cl2=5KCl+KClO3+3H2O(�������¶Ƚϸ�)
��K2FeO4��ˮ��Һ����ˮ�⣺4FeO42-+10H2O![]() 4Fe(OH)3(����)+8OH-+3O2��
4Fe(OH)3(����)+8OH-+3O2��
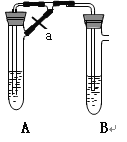
�ش��������⣺ʵ���ҿ�������ͼװ��������̢ٺ͢�

��1��д����ҵ����ȡCl2�Ļ�ѧ����ʽ__����ˮ���е�ˮΪ___(������ˮ��������ˮ��)��
��2����Ӧһ��ʱ���ֹͣͨ��������������a�м���ŨKOH��Һ��Ŀ����___��
A��Ϊ��һ����Ӧ�ṩ���ԵĻ���
B��ʹKClO3ת��Ϊ KClO
C������ҺI�й�����Cl2������Ӧ�����ɸ����KClO
D��KOH�����ܽ�ʱ��ų��϶����������������߷�Ӧ����
��3������Һ���з���� K2FeO4�����и���ƷKNO3��KCl����Ӧ���з��������ӷ���ʽΪ��___��
��4�����ؽᾧ���ᴿ�ֲ�Ʒ�����ֲ�Ʒ����KOHϡ��Һ�ܽ⣬�ټ��뱥�͵�KOH��Һ����ȴ�ᾧ�����ˣ������������ϴ�ӣ���������ո��
��ϴ�Ӵ�Ʒʱѡ�������������ˮ��������___��
������ж� K2FeO4 �����Ѿ�ϴ�Ӹɾ�__��
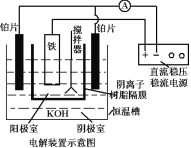
��5���ӻ��������ĽǶȿ����Ʊ�K2FeO4�Ϻõķ���Ϊ��ⷨ����װ����ͼ���������������ĵ缫��ӦʽΪ___��
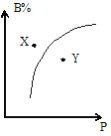
��6��FeO42-��ˮ��Һ�еĴ�����̬��ͼ��ʾ������˵����ȷ����___��
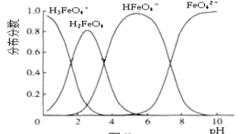
A��������Һ���������α仯����Ԫ�ض���4 �ִ�����̬
B����pH=10��������Һ�м�������pH=2��HFeO4-�ķֲ�����������
C����pH=6 ��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪHFeO4-+OH-=FeO42- +H2O
���𰸡�2NaCl+2H2O![]() 2NaOH+H2��+Cl2�� ��ˮ AC 2Fe3+ +3ClO-+10OH-=2FeO42-+3Cl-+5H2O ��ֹFeO42-ˮ�⣬���ں�� ȡ�������һ�ε�ϴ��Һ�����������ữ����������Һ�����ް�ɫ�������ѱ�ϴ�� Fe+8OH--6e-�TFeO42-+4H2O C
2NaOH+H2��+Cl2�� ��ˮ AC 2Fe3+ +3ClO-+10OH-=2FeO42-+3Cl-+5H2O ��ֹFeO42-ˮ�⣬���ں�� ȡ�������һ�ε�ϴ��Һ�����������ữ����������Һ�����ް�ɫ�������ѱ�ϴ�� Fe+8OH--6e-�TFeO42-+4H2O C
��������
��1����ҵ����ȡCl2�ǵ�ⱥ��ʳ��ˮ����Ӧ�Ļ�ѧ����ʽ2NaCl+2H2O![]() 2NaOH+H2��+Cl2������Ϊ��2NaCl+2H2O
2NaOH+H2��+Cl2��������2NaCl+2H2O![]() 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����
��ΪҪ���NaClO������Ӧ���Ʒ�ӦҺ���¶ȣ���ˮ���е�ˮΪ��ˮ����Ϊ����ˮ��
��2����Ӧһ��ʱ���ֹͣͨ��������������a�м���ŨKOH��Һ��Ŀ���ǣ�
һ����Ҫ���ǹ���Cl2�Ĵ�������һ����Ҫ���Ǻ�����Ӧ����Ҫ������Ի�����
����AC��
��3������Һ���з���� K2FeO4�����и���ƷKNO3��KCl����Ӧ���з��������ӷ���ʽΪ��2Fe3+ +3ClO-+10OH-=2FeO42-+3Cl-+5H2O��
����2Fe3+ +3ClO-+10OH-=2FeO42-+3Cl-+5H2O��
��4�����ؽᾧ���ᴿ�ֲ�Ʒ�����ֲ�Ʒ����KOHϡ��Һ�ܽ⣬�ټ��뱥�͵�KOH��Һ����ȴ�ᾧ�����ˣ������������ϴ�ӣ���������ո��
����ΪK2FeO4��ˮ��Һ����ˮ�⣬����ϴ�Ӵ�Ʒʱѡ�������������ˮ�������Ƿ�ֹFeO42-ˮ�⣬���ں�ɡ�
��Ϊ����ֹFeO42-ˮ�⣬���ں�ɣ�
���ж�K2FeO4 �����Ѿ�ϴ�Ӹɾ�������Ҫ���龧������Ƿ�������Һ�е�Cl-������Ӧʹ�������ữ����������Һ��
��Ϊ��ȡ�������һ�ε�ϴ��Һ�����������ữ����������Һ�����ް�ɫ�������ѱ�ϴ����
��5���Ʊ�K2FeO4�Ϻõķ���Ϊ��ⷨ������Ϊ�����ڼ�����Һ������FeO4-,�缫��ӦʽΪFe+8OH--6e-�TFeO42-+4H2O����Ϊ��Fe+8OH--6e-�TFeO42-+4H2O��
��6��A����ͼ�п��Կ�������Ԫ���ڲ�ͬpH��Χ�ڣ�������̬��ͬ��A����
B����pH=10��������Һ�м�������pH=2��HFeO4-�ķֲ����������������С��B����
C����pH=6 ��������Һ�м�KOH��Һ��������Ӧ�����ӷ���ʽΪHFeO4-+OH-=FeO42- +H2O��C��ȷ��
��ѡC��

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�