��Ŀ����
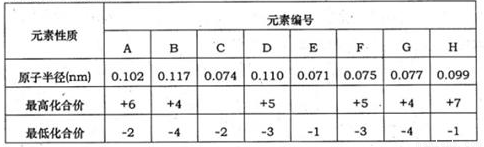
��10�֣��±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ���Ӧ�⻯��е�����ݣ�
|
Ԫ������ |
Ԫ�ر�� |
|||||||
|
A |
B |
C |
D |
E |
F |
G |
H |
|
|
�⻯��ķе㣨�棩 |
��60.7 |
��33.4 |
��111.5 |
100 |
��87.7 |
19.54 |
��84.9 |
��161.5 |
|
����ϼ� |
+6 |
+5 |
��4 |
|
+5 |
|
+7 |
��4 |
|
��ͻ��ϼ� |
��2 |
��3 |
��4 |
��2 |
��3 |
��1 |
��1 |
��4 |
��֪����A��D���γɻ�����AD2��AD3���������Ʊ�ǿ��ף�
��B��D���γɻ�����BD��BD2���������Ʊ�ǿ���ҡ�
��ش�
��1���������ڵ�������Ԫ�ص��� ���ñ���Ԫ�ر����д����
��2��д��H�����������ĵ���ʽ�� ��
�Ƚ�A��D��G���ּ������ӵİ뾶��С��r( )��r( )��r( ) ������ʵ�ʵ�Ԫ�ط��ű�ʾ����
��3���ɱ���DԪ�غ���Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�________________��
a��MnO2 b��FeCl3 c��Na2SO3 d��KMnO4
��4���������ΪADG2��������ˮ�л�ǿ��ˮ�⣬����ʹƷ����Һ��ɫ����ɫ�����һ��ǿ�ᡣ�÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��

����������



 �±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺