��Ŀ����
9����NH4��2SO4�dz��õĻ��ʺͻ���ԭ�ϣ������ֽ⣮ij��ȤС����̽����ֽ������������ϡ���NH4��2SO4��260���400��ʱ�ֽ���ﲻͬ��
��ʵ��̽������С����ѡ����ͼ��ʾװ�ý���ʵ�飨�гֺͼ���װ���ԣ�
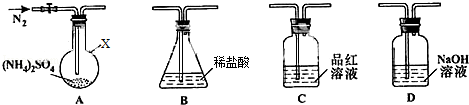
ʵ��1������װ��A-B-C-D����������ԣ���ͼʾ�����Լ���װ��Bʢ0.5000mol/L���� 70.00mL��ͨ��N2�ž���������260�����װ��Aһ��ʱ�䣬ֹͣ���ȣ���ȴ��ֹͣͨ��N2��Ʒ����Һ����ɫ��ȡ��װ��B������ָʾ������0.2000moI/LNaOH��Һ�ζ�ʣ�����ᣬ�յ�ʱ����NaOH��Һ25.00mL��������ζ������Һ����SO42-��
��1������X��������Բ����ƿ��
��2���ζ�ǰ�����в�������ȷ˳����dbaec������ĸ��ţ���
a��ʢװ 0.2000mol/LNaOH��Һ
b����0.2000mol/L NaOH��Һ��ϴ
c����������¼
d����©����ϴ
e���ž��ζ���������ݲ�����Һ��
��3��װ��B����Һ������������ʵ�����0.03mol��
ʵ��2������װ��A-D-B����������ԣ���ͼʾ���¼����Լ���ͨ��N2�ž���������400�����װ��A����NH4��2SO4��ȫ�ֽ������ֹͣ���ȣ���ȴ��ֹͣͨ��N2���۲쵽װ��A��D֮��ĵ���������������ɫ���壮�����飬�ð�ɫ�����װ��D����Һ����SO32-��SO42-����һ���о����֣�����������������
��4�����װ��D����Һ����SO32-����SO42-��ʵ�������������ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��B����Һ���յ�������NH3��
��6����NH4��2SO4��400��ֽ�Ļ�ѧ����ʽ��3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
���� ��1����ͼ������������֪XΪԲ����ƿ��
��2���ζ�ǰ���ȼ��ζ����Ƿ�©ˮ���ٽ�����ϴ��Ȼ���ñ�Һ��ϴ����ע���Һ���ž��ζ���������ݲ�����Һ�棬��������¼���ζ�ǰ����ɣ�
��3�����������������Ƽ���Bװ����ʣ���HCl���μӷ�Ӧ��HCl���շֽ����ɵ�NH3��������Ӧ��NH3+HCl=NH4Cl��������������NH3�����ʵ�����
��4��ȡD��Һ���Թ��У���������BaCl2��Һ���ټ������ᣬ��ɫ������ȫ�ܽ������ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��D����Һ����SO32-��˵���ֽ�����SO2��װ��A��D֮��ĵ���������������ɫ���壬��ɫ����Ӧ�Ƕ�����������ˮ�γɵ��Σ�װ��B����Һ���յ������ǰ�����
��6���ɣ�5���з�����֪����NH4��2SO4��400��ֽ�ʱ����NH3��SO2��H2O���ɣ�SԪ�ػ��ϼ۽��ͣ����ݵ���ת���غ㣬ֻ��ΪNԪ�ػ��ϼ����ߣ�����������������˵������N2����ƽ��д����ʽ��
��� �⣺��1��������X�Ľṹ��֪��XΪԲ����ƿ���ʴ�Ϊ��Բ����ƿ��
��2���ζ�ǰ���ȼ��ζ����Ƿ�©ˮ���ٽ�����ϴ��Ȼ���ñ�Һ��ϴ����ע���Һ���ž��ζ���������ݲ�����Һ�棬��������¼���ζ�ǰ����ɣ�����ȷ��˳��Ϊ��dbaec��
�ʴ�Ϊ��dbaec��
��3���ζ�ʣ�����ᣬ�յ�ʱ����NaOHΪ0.025L��0.2mol/L=0.005mol����ʣ��HClΪ0.005mol����μӷ�Ӧ��HClΪ0.07L��0.5mol/L-0.005mol=0.03mol���μӷ�Ӧ��HCl���շֽ����ɵ�NH3��������Ӧ��NH3+HCl=NH4Cl��������NH3�����ʵ���Ϊ0.03mol��
�ʴ�Ϊ��0.03mol��
��4�����װ��D����Һ����SO32-����SO42-��ʵ������������ǣ�ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
�ʴ�Ϊ��ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��D����Һ����SO32-��˵���ֽ�����SO2��װ��A��D֮��ĵ���������������ɫ���壬��ɫ����Ӧ�Ƕ�����������ˮ�γɵ��Σ�װ��B����Һ���յ������ǰ�����
�ʴ�Ϊ��NH3��
��6���ɣ�5���з�����֪����NH4��2SO4��400��ֽ�ʱ����NH3��SO2��H2O���ɣ�SԪ�ػ��ϼ۽��ͣ����ݵ���ת���غ㣬ֻ��ΪNԪ�ػ��ϼ����ߣ�����������������˵������N2���ֽⷴӦ����ʽΪ3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
�ʴ�Ϊ��3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
���� ���⿼������ʵ�鷽������ƣ�Ϊ��Ƶ���㣬���շ�Ӧԭ����ʵ�鲽�輰ʵ��װ�õ�����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | ���ˮ�еμӱ��͵��Ȼ�����Һ��ȡ���壺Fe3++3H2O�TFe��OH��3��+3H+ | |
| B�� | ̼����þ��Һ�м�������������������Һ��Mg2++2HCO3-+2OH-�TMgCO3��+CO32-+2H2O | |
| C�� | ��������Һ�м�������İ�ˮ��Al3++4NH3+2H2O�TAlO2-+4NH4+ | |
| D�� | ���۵⻯����ֽ�ڳ�ʪ�����б�����4I-+O2+2H2O�T2I2+4OH- |
| A�� | �����ʵ�����N2��CO������������ΪNA | |
| B�� | 1.7gH2O2�к��еĵ�����Ϊ0.9NA | |
| C�� | 10mL��������98%��H2SO4����ˮϡ����100mL��H2SO4����������Ϊ9.8% | |
| D�� | 1molCl2������������Ӧ��ת�Ƶĵ�����Ϊ3NA |
| A�� | S��P | B�� | Mg��Al | C�� | Na��Mg | D�� | Ne��He |
| A�� | ��ϩ��3��1��ϩ | B�� | ������Ȳ | ||
| C�� | 1�ȱ����2�ȱ��� | D�� | �ױ����ұ� |
 ��ͼ��M��N�������ʵ��ܽ�����ߣ�t2��ʱ��ʢ��100gˮ���ձ����Ⱥ����ag M��ag N�����������ܽ�ʱ����Ӱ�죬����������M��N������ֽ��裮������t1�棬����˵����ȷ���ǣ�������
��ͼ��M��N�������ʵ��ܽ�����ߣ�t2��ʱ��ʢ��100gˮ���ձ����Ⱥ����ag M��ag N�����������ܽ�ʱ����Ӱ�죬����������M��N������ֽ��裮������t1�棬����˵����ȷ���ǣ�������| A�� | t2��ʱ���õ�M�ı�����Һ | |
| B�� | t2��ʱ���õ�N�IJ�������Һ | |
| C�� | �¶Ƚ��͵�t1��ʱ���õ�M��N�IJ�������Һ������Һ�����ʵ������������ | |
| D�� | �¶Ƚ��͵�t1��ʱ��M��N���ܽ����ȣ��õ�M��N�ı�����Һ |
| A�� | ��������һ�������ɸ�CH2ԭ���ţ��ҷ���ͬһͨʽ���л���֮�以��Ϊͬϵ�� | |
| B�� | ���ۡ���ά�ء���Ȼ��֬�ȶ����ڻ���� | |
| C�� | ���ڱ����ӽṹ�������ԣ���˱����ܷ����ӳɷ�Ӧ | |
| D�� | �������������ԭ�Ӳ���һ��ƽ���ϣ���3��̼ԭ����һֱ���� |
�����ǵؿ��ﺬ�����Ľ���Ԫ�آڵ���������Ŀǰ���ڱ��к�Ԫ����������
��Na�Ƕ�����Ԫ����ԭ�Ӱ뾶��������Ԫ�� ��F�Ƿǽ�����ǿ��Ԫ�أ�
| A�� | ȫ�� | B�� | ������ȫ�� | C�� | ������ȫ�� | D�� | ������ȫ�� |