��Ŀ����
��1��������ͬ��O2��NH3��H2��Cl2���������У�����������Ŀ���ٵ��� ������ͬ�¶Ⱥ���ͬѹǿ�����£���������� ��
��2������100ml��0.2mol/L��CuSO4��Һ����ҪCuSO4?5H2O������ g��
��3��ʵ����Ҫ90ml��2mol/LKCl��Һ��������KCl�������ƣ�����������ƽ��ȡ g KCl
��4��ʵ�����ﳣ��KMnO4��Ũ���ᷴӦ��ȡ�����������仯ѧ����ʽΪ��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O
�����������11.2L Cl2�������ת�Ƶ���Ŀ ��
��2������100ml��0.2mol/L��CuSO4��Һ����ҪCuSO4?5H2O������
��3��ʵ����Ҫ90ml��2mol/LKCl��Һ��������KCl�������ƣ�����������ƽ��ȡ
��4��ʵ�����ﳣ��KMnO4��Ũ���ᷴӦ��ȡ�����������仯ѧ����ʽΪ��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O
�����������11.2L Cl2�������ת�Ƶ���Ŀ
���㣺���ʵ�������ؼ���,������ԭ��Ӧ
ר�⣺������
��������1������n=
��֪��������������£�Ħ������Խ�����ʵ���ԽС����N=nNA��֪�����ʵ���Խ���з�����ĿԽ��ͬ��ͬѹ�£��������֮�ȵ������ʵ���֮�ȣ�
��2������n=cV��������ͭ���ʵ�����CuSO4��CuSO4?5H2O�����ʵ�����ȣ�����m=nM��������ͭ����������
��3��û��90mL����ƿ��Ӧѡ��100mL����ƿ������n=cV����KCl���ʵ������ٸ���m=nM������������
��4������n=
�����������ʵ��������ClԪ�ػ��ϼ۱仯����ת�Ƶ�����Ŀ��
| m |
| M |
��2������n=cV��������ͭ���ʵ�����CuSO4��CuSO4?5H2O�����ʵ�����ȣ�����m=nM��������ͭ����������
��3��û��90mL����ƿ��Ӧѡ��100mL����ƿ������n=cV����KCl���ʵ������ٸ���m=nM������������
��4������n=
| V |
| Vm |
���
�⣺��1��Ħ��������Cl2��O2��NH3��H2������n=
��֪��������������£�Cl2���ʵ�����С��H2���ʵ������
��N=nNA��֪�����ʵ���Խ���з�����ĿԽ������������Ŀ���ٵ���Cl2��ͬ��ͬѹ�£��������֮�ȵ������ʵ���֮�ȣ������������Cl2��
�ʴ�Ϊ��Cl2��H2��
��2������ͭ���ʵ���=0.1L��0.2mol/L=0.02mol��CuSO4��CuSO4?5H2O�����ʵ�����ȣ���Ҫ����ͭ��������=0.02mol��250g/mol=5.0g��
�ʴ�Ϊ��5.0��
��3��û��90mL����ƿ��Ӧѡ��100mL����ƿ��KCl���ʵ���=0.1L��2mol/L=0.2mol����ҪKCl����=0.2mol��74.5g/mol=14.9g��
�ʴ�Ϊ��14.9��
��4������£�11.2L ���������ʵ���=
=0.5mol����ӦClԪ�ػ��ϼ���-1������Ϊ0�ۣ���ת�Ƶ�����Ŀ=0.5mol��2��6.02��1023mol-1=6.02��1023��
�ʴ�Ϊ��6.02��1023��
| m |
| M |
��N=nNA��֪�����ʵ���Խ���з�����ĿԽ������������Ŀ���ٵ���Cl2��ͬ��ͬѹ�£��������֮�ȵ������ʵ���֮�ȣ������������Cl2��
�ʴ�Ϊ��Cl2��H2��
��2������ͭ���ʵ���=0.1L��0.2mol/L=0.02mol��CuSO4��CuSO4?5H2O�����ʵ�����ȣ���Ҫ����ͭ��������=0.02mol��250g/mol=5.0g��
�ʴ�Ϊ��5.0��
��3��û��90mL����ƿ��Ӧѡ��100mL����ƿ��KCl���ʵ���=0.1L��2mol/L=0.2mol����ҪKCl����=0.2mol��74.5g/mol=14.9g��
�ʴ�Ϊ��14.9��
��4������£�11.2L ���������ʵ���=
| 11.2L |
| 22.4L/mol |
�ʴ�Ϊ��6.02��1023��
���������⿼�����ʵ����йؼ��㣬�ѶȲ���ע��Թ�ʽ�����������Ӧ�ã���3��Ϊ�״��㣬ѧ������������ƿ�Ĺ��������Һ������㣬���´���

��ϰ��ϵ�д�
�����Ŀ
��ȡFe��OH��2���ܽϳ�ʱ�䱣�ְ�ɫ����ѡȡ�IJ����У��ټ���һЩֲ���ͣ�������ȴ������ˮ�ܽ�FeSO4�������Һ���۰�����ˮ������в��ܷ���ȴ���ܼ���������м���ݼ�������CCl4����ͷ�ιܴ�ֱ�Թܿ���FeSO4��Һ�е���������ˮ���߰�ʢ�а�ˮ�Ľ�ͷ�ι�����FeSO4��Һ�к��ټ�����ˮ�����б�����еIJ�����������˳����ȷ���ǣ�������
| A���ۢڢݢޢ� |
| B���ۢڢܢ٢� |
| C���ۢڢܢݢ� |
| D���ڢܢ٢ߢ� |
����˵������ȷ���ǣ�������
| A�����н���Ԫ�ص�����һ���������ӣ����������ӱ���ԭһ���õ��������� |
| B��pH��ȵ�NaOH��NaHCO3��Na2CO3������Һ��c��NaOH����c��NaHCO3����c��Na2CO3�� |
| C�������£�c��NH4+����ȵ�4����Һ���٣�NH4��2SO4 �ڣ�NH4��2Fe��SO4��2 ��NH4Cl �ܣ�NH4��2CO3���������ʵ���Ũ�ȴ�С��ϵ�ǣ��ڣ��٣��ܣ��� |
| D��ij�¶�ʱˮ�����ӻ�����KW=10-13�������¶���pH=11��NaOH��Һa L��pH=1��ϡ����b L��ϣ������û��ҺpH=2����a��b=2��9 |
 1Lij�����Һ�����ܺ��е��������±���
1Lij�����Һ�����ܺ��е��������±���
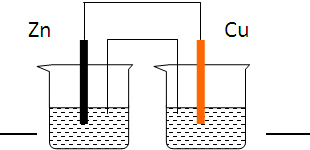 �ܷ�Ӧ��Zn+Cu2+=Zn2++Cu��
�ܷ�Ӧ��Zn+Cu2+=Zn2++Cu��