��Ŀ����
����Ŀ���ҹ������з��ġ�����һ�š����й��Ϻ����������ɿ�ȼ�������Բɣ����õ��й��������Ժ�����µ�ף�ء�����ȼ��������Ȼ��ˮ�������������ڳ��³�ѹ��Ѹ�ٷֽ��ͷų����飬����Ϊδ������Դ��
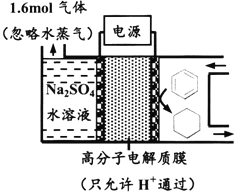
��1������ȼ������Ϊ��Դ���ŵ���_______________________________(�ش�һ������)��
��2����������������һ���Ƚ������ⷽ��,�����������������������������̡���Ӧϵͳͬʱͨ����顢������ˮ����,��������Ҫ��ѧ��Ӧ����:
��Ӧ���� | ��ѧ����ʽ | �ʱ��H(kJ.mol-l) | ���E.(kJ.mol-1) |
�������� | CH4(g)+ | -802.6 | 125.6 |
CH4(g)+O2(g) | -322.0 | 172.5 | |
�������� | CH4(g)+H2O(g) | +206.2 | 240.1 |
CH4(g)+2H2O(g) | +158. 6 | 243.9 |
�ش���������:
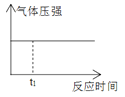
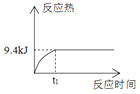
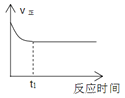
���ڳ�ʼ�Σ��������������ķ�Ӧ����______(����ڡ���С�ڡ����ڡ�)���������ķ�Ӧ���ʡ�
�ڷ�ӦCH4(g)+H2O(g)![]() CO(g)+3H2(g)��ƽ��ת�������¶ȡ�ѹǿ��ϵ[����n(CH4)��n(H2O)=1:1]��ͼ��ʾ��
CO(g)+3H2(g)��ƽ��ת�������¶ȡ�ѹǿ��ϵ[����n(CH4)��n(H2O)=1:1]��ͼ��ʾ��

�÷�Ӧ��ͼ��A���ƽ�ⳣ��Kp=________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��ͼ��ѹǿ(p1��p2��p3��p4)�ɴ�С��˳��Ϊ___________��
�۴������Ƕȷ������������������������Ƚ�֮������___________��
��3�����鳬������CO2�����ɵõ�����CO�����壬����Դ�ͻ����ϵ�˫�������ش��鳬������CO2�Ĵ�ת��ԭ����ͼ��ʾ��

�ٹ���II�еڶ�����Ӧ�Ļ�ѧ����ʽΪ_________��
�ڹ���II�Ĵ�����______��ֻ�й���IͶ�ϱ�![]() _______��������ɲŻᱣ�ֲ��䡣
_______��������ɲŻᱣ�ֲ��䡣
���𰸡������ܶȸߡ���ࡢ��ȾС�������� С�� 3/16(Mpa)2��0.1875( Mpa)2 p1>p2>p3>p4 ����������Ӧ�ų�����������������������Ӧ���������������ﵽ����ƽ�� 3Fe+4CaCO3![]() Fe3O4+4CaO+4CO�� Fe3O4��CaO 1/3
Fe3O4+4CaO+4CO�� Fe3O4��CaO 1/3
��������
��1������ȼ������һ�ְ�ɫ���壬�м�ǿ��ȼ������
��2���ٴӱ��л�����ݿ����ڳ�ʼ�Σ���������������Ӧ��ܽϴ����������ķ�Ӧ��ܾ���С�����Լ��������ķ�Ӧ���ʿ졣
�ڼ��������1mol��ˮ������1mol��
CH4(g)+H2O(g)![]() CO(g)+3H2(g)
CO(g)+3H2(g)
��ʼ�� 1 1 0 0
�仯�� 0.2 0.2 0.2 0.6
ƽ���� 0.8 0.8 0.2 0.6
��������ʽ���з�����ɡ�
�ۼ���������Ӧ�ų����������ṩ����������������Ӧ�����������������ﵽ������á�
��3����.����ͼʾ��������һ����Ӧ�ǻ�ԭ����������������ԭΪ�����ڶ�����Ӧ������̼�������Ϊ��������������������ԭΪһ����̼��
��.��Ӧ�����̣���CH4(g)+CO2(g)![]() 2CO(g)+2H2(g)����4H2(g)+ Fe3O4(s)
2CO(g)+2H2(g)����4H2(g)+ Fe3O4(s) ![]() 3Fe(s)+4 H2O(g)����Fe3O4(s)+4CO(g)
3Fe(s)+4 H2O(g)����Fe3O4(s)+4CO(g) ![]() 3Fe(s)+ 4CO2(g)��������Ӧ��ȥFe3O4��Fe�����յõ�CH4(g)+3CO2(g)
3Fe(s)+ 4CO2(g)��������Ӧ��ȥFe3O4��Fe�����յõ�CH4(g)+3CO2(g) ![]() 2H2O(g)+ 4CO(g)��ֻ�й���IͶ�ϱ�
2H2O(g)+ 4CO(g)��ֻ�й���IͶ�ϱ�![]() ��������ɲŻᱣ�ֲ��䡣
��������ɲŻᱣ�ֲ��䡣
��1������ȼ������һ�ְ�ɫ���壬�м�ǿ��ȼ�������ŵ��ǣ������ܶȸߡ���ࡢ��ȾС�������ʴ�Ϊ�������ܶȸߡ���ࡢ��ȾС��������
��2���ٴӱ��л�����ݿ����ڳ�ʼ�Σ���������������Ӧ��ܽϴ����������ķ�Ӧ��ܾ���С�����Լ��������ķ�Ӧ���ʿ죬�ʴ�Ϊ��С�ڡ�
�ڼ��������1mol��ˮ������1mol��
CH4(g)+H2O(g)![]() CO(g)+3H2(g)
CO(g)+3H2(g)
��ʼ�� 1 1 0 0
�仯�� 0.2 0.2 0.2 0.6
ƽ���� 0.8 0.8 0.2 0.6
ƽ����������������0.8 + 0.8 + 0.2 + 0.6=2.4mol��p(CH4)= p(H2O)=4/3��p(CO)=1/3��p(H2)=1��A���ƽ�ⳣ��Kp=13��1/3��(4/3)2=3/16(Mpa)2,����ͼ��������¶Ȳ���ʱ��ѹǿ��С��ƽ�����ƣ������ת��������ѹǿ(p1��p2��p3��p4)�ɴ�С��˳��Ϊ��p1>p2>p3>p4���ʴ�Ϊ��3/16(Mpa)2��0.1875( Mpa)2 ��p1>p2>p3>p4 ��
�ۼ���������Ӧ�ų����������ṩ����������������Ӧ�����������������ﵽ������ã��ʴ�Ϊ������������Ӧ�ų�����������������������Ӧ���������������ﵽ����ƽ�⡣
��3����.����ͼʾ��������һ����Ӧ�ǻ�ԭ����������������ԭΪ�����ڶ�����Ӧ������̼�������Ϊ��������������������ԭΪһ����̼���ʴ�Ϊ��3Fe+4CaCO3![]() Fe3O4+4CaO+4CO����
Fe3O4+4CaO+4CO����
��.����II�Ĵ����ǣ�Fe3O4��CaO����Ӧ�����̣���CH4(g)+CO2(g)![]() 2CO(g)+2H2(g)����4H2(g)+ Fe3O4(s)
2CO(g)+2H2(g)����4H2(g)+ Fe3O4(s) ![]() 3Fe(s)+4 H2O(g)����Fe3O4(s)+4CO(g)
3Fe(s)+4 H2O(g)����Fe3O4(s)+4CO(g) ![]() 3Fe(s)+ 4CO2(g)��������Ӧ��ȥFe3O4��Fe�����յõ�CH4(g)+3CO2(g)
3Fe(s)+ 4CO2(g)��������Ӧ��ȥFe3O4��Fe�����յõ�CH4(g)+3CO2(g) ![]() 2H2O(g)+ 4CO(g)��ֻ�й���IͶ�ϱ�
2H2O(g)+ 4CO(g)��ֻ�й���IͶ�ϱ�![]() ��������ɲŻᱣ�ֲ��䡣�ʴ�Ϊ��Fe3O4��CaO ��1/3��
��������ɲŻᱣ�ֲ��䡣�ʴ�Ϊ��Fe3O4��CaO ��1/3��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�