��Ŀ����
����Ŀ��X��Y��Z��W ���������������ת����ϵ������ Y��Z Ϊ�����δ�г���Ӧ��������

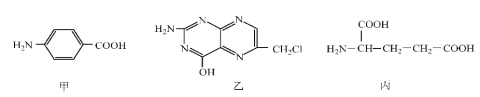
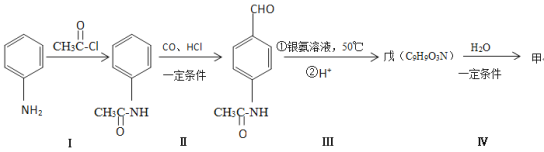
��1����ʵ���Ҿ����ó���ʯ��ˮ������ X ��ȼ�ղ��W ����;֮һ�Ǽ����оƬ��W �����ڱ��е�λ��Ϊ ___________��Y ����;�� _________��д��Y��NaOH ��Һ��Ӧ�����ӷ���ʽ _________��
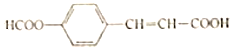
��2���� X��W Ϊ�ճ������г��������ֽ������� Y ��������ɫ�����Ϳ�ϣ���÷�Ӧ�Ļ�ѧ����ʽΪ___________��
��3���� X Ϊ����ɫ��ĩ��Y Ϊ�����г���Һ�壬��
��X �ĵ���ʽΪ _______________���÷�Ӧ�Ļ�ѧ����ʽΪ ____________�����ɵĻ�������������ѧ�������� ________________________��
�� �� 7.8 �� X ������ȫ��Ӧ��ת�Ƶĵ�����Ϊ ___________��
���𰸡��������� ��A�� ���ά SiO2+2OH-=SiO32-+H2O ![]()
![]() 2Na2O2��2H2O=4NaOH��O2�� ���Ӽ������Լ� 0.1NA
2Na2O2��2H2O=4NaOH��O2�� ���Ӽ������Լ� 0.1NA
��������
����ʵ���Ҿ����ó���ʯ��ˮ������ X ��ȼ�ղ����XΪ̼��W ����;֮һ�Ǽ����оƬ����Ϊ�裬W �����ڱ��е�λ��Ϊ�������ڵ�IVA�壬YΪ�������裬������;�� ���ά��д��Y��NaOH ��Һ��Ӧ�����ӷ���ʽSiO2+2OH-=SiO32��+H2O��
�ʴ�Ϊ����������A�壻���ά��SiO2+2OH-=SiO32��+H2O��
����X��WΪ�ճ������г��������ֽ�������Y��������ɫ�����Ϳ�ϼ�Ϊ�����������߷������ȷ�Ӧ����÷�Ӧ�Ļ�ѧ����ʽΪ2Al + Fe2O3 ![]() 2Fe+ Al2O3��
2Fe+ Al2O3��
�ʴ�Ϊ2Al + Fe2O3 ![]() 2Fe+ Al2O3��
2Fe+ Al2O3��
����XΪ����ɫ��ĩ��Ϊ�������ƣ�Y Ϊ�����г���Һ�弴Ϊ ����
��X�ĵ���ʽΪ![]() ���÷�Ӧ�Ļ�ѧ����ʽΪ2Na2O2��2H2O=4NaOH��O2�������ɵĻ�����NaOH������ѧ�����������Ӽ������Լ���
���÷�Ӧ�Ļ�ѧ����ʽΪ2Na2O2��2H2O=4NaOH��O2�������ɵĻ�����NaOH������ѧ�����������Ӽ������Լ���
�ʴ�Ϊ![]() ��2Na2O2��2H2O=4NaOH��O2�������Ӽ������Լ���
��2Na2O2��2H2O=4NaOH��O2�������Ӽ������Լ���
������������һ��������һ�ۣ�һ��������һ�ۣ���7.8�˹������Ƽ�0.1 mol��ȫ��Ӧ��ת�Ƶĵ�����Ϊ0.1NA��
�ʴ�Ϊ0.1NA��

 ������������ϵ�д�
������������ϵ�д�����Ŀ��Ϊ��ǿ������ʴ��,����Ǧ����Ϊ���Դ,��Al��������Pb������,���ϡ����,ʹ�����������Ĥ����Ӧԭ������:
���:Pb(s)+PbO2(s)+2H2SO4(aq)=2PbSO4(s)+2H2O(l)
����:2Al+3H2O![]() Al2O3+3H2��
Al2O3+3H2��
��������,�����ж���ȷ����( )
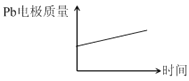
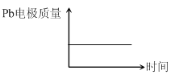
��� | ���� | |
A | H+����Pb�缫 | H+����Pb�缫 |
B | ÿ����3molPb | ����2molAl2O3 |
C | ����:PbO2+4H++2e-=Pb2++2H2O | ����:2Al+3H2O-6e-=Al2O3+6H+ |
D |
|
|
A. AB. BC. CD. D