��Ŀ����
��10�֣����Ṥҵ�ڹ��á�������ҵ�ͺ�����ҵ��ռ����Ҫ��λ�����Ż�ѧ��ά�������л��ϳɡ�����������ȹ�ҵ��Ѹ�ٷ�չ����Ҫʹ�ô��������ᡣ
��1����ҵ�������ò�ϵ������Ϊ�������ð���������ȡ���ᡣд���ò���Ͻ��������ɰ������Ʊ�����ĵ�һ����Ӧ�Ļ�ѧ����ʽ �� ��Ũ�����Ũ����Ļ�������л��ϳ��г��õ���������д����������Ũ�����Ũ������Һ�У�����50��--60�淴Ӧ�Ļ�ѧ����ʽ �� ��
��2��ͭ������ϡ���ᷴӦ��Ҳ����Ũ���ᷴӦ����ͭ��һ��Ũ�����ᷴӦʱ���ɽ�����ʽ��ʾΪ��Cu+HNO3 �� Cu(NO3)2+NO��+NO2��+H2O ������ʽδ��ƽ�����������ڸ÷�Ӧ�е������� �� ������õ���NO��NO2���ʵ�����ͬ��д������ƽ�÷�Ӧ�����ӷ���ʽ �� ��0.6mol Cu��������ȫ�ܽ��������ˮ���ռ���Щ���壬�ɵñ�״���µ��������Ϊ �� ��
��1����ҵ�������ò�ϵ������Ϊ�������ð���������ȡ���ᡣд���ò���Ͻ��������ɰ������Ʊ�����ĵ�һ����Ӧ�Ļ�ѧ����ʽ �� ��Ũ�����Ũ����Ļ�������л��ϳ��г��õ���������д����������Ũ�����Ũ������Һ�У�����50��--60�淴Ӧ�Ļ�ѧ����ʽ �� ��
��2��ͭ������ϡ���ᷴӦ��Ҳ����Ũ���ᷴӦ����ͭ��һ��Ũ�����ᷴӦʱ���ɽ�����ʽ��ʾΪ��Cu+HNO3 �� Cu(NO3)2+NO��+NO2��+H2O ������ʽδ��ƽ�����������ڸ÷�Ӧ�е������� �� ������õ���NO��NO2���ʵ�����ͬ��д������ƽ�÷�Ӧ�����ӷ���ʽ �� ��0.6mol Cu��������ȫ�ܽ��������ˮ���ռ���Щ���壬�ɵñ�״���µ��������Ϊ �� ��
��10�֣�
(1) 4NH3+5O2 4NO+6H2O, ��2�֣�

��2�֣�
(2) ��1������������ ��2�֣�
��3��2Cu��6H����2NO3��=2Cu2����NO����NO2����3H2O ��2�֣�8.96 L ��2�֣�
(1) 4NH3+5O2 4NO+6H2O, ��2�֣�

��2�֣�
(2) ��1������������ ��2�֣�
��3��2Cu��6H����2NO3��=2Cu2����NO����NO2����3H2O ��2�֣�8.96 L ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
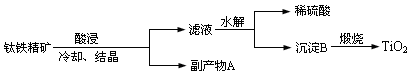



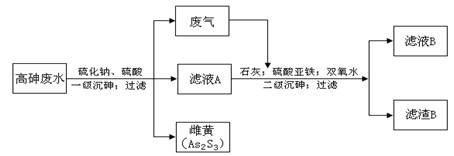
 ���ͣ������ա�
���ͣ������ա�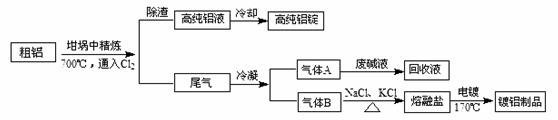
 ��NaCl�۵�Ϊ801�棻AlCl3��181��������
��NaCl�۵�Ϊ801�棻AlCl3��181��������