��Ŀ����
��12�֣��������������������ˮ��ҵ��ˮ������������������Ͽ졣ʵ���ҿ������̿���Ҫ�ɷ�MnO2��Ϊԭ���Ʊ�������ء��䲿���������£�

��1��ʵ���в��������������ô�������ԭ���� ��
��2��KOH��KClO3��MnO2�����Ƶ���ɫK2MnO4�Ļ�ѧ����ʽ ��
��3��ͨ��CO2����ʹMnO42�������绯��Ӧ������MnO4����MnO2����K2MnO4��ɷ�Ӧʱ��ת��ΪKMnO4�İٷ���Լ ����ȷ��0��1����
��4����ͨ��CO2̫�࣬���ڼ���Ũ��ʱ������ �����KMnO4����һ��������
��5���ڼ��ȡ�Ũ������ɵĹ������¶Ȳ��˹��ߣ���ԭ���� ������ʱ�ò�������ͣ������Һ��Ŀ���� ��

��1��ʵ���в��������������ô�������ԭ���� ��
��2��KOH��KClO3��MnO2�����Ƶ���ɫK2MnO4�Ļ�ѧ����ʽ ��
��3��ͨ��CO2����ʹMnO42�������绯��Ӧ������MnO4����MnO2����K2MnO4��ɷ�Ӧʱ��ת��ΪKMnO4�İٷ���Լ ����ȷ��0��1����
��4����ͨ��CO2̫�࣬���ڼ���Ũ��ʱ������ �����KMnO4����һ��������
��5���ڼ��ȡ�Ũ������ɵĹ������¶Ȳ��˹��ߣ���ԭ���� ������ʱ�ò�������ͣ������Һ��Ŀ���� ��

��

��ϰ��ϵ�д�
�����Ŀ
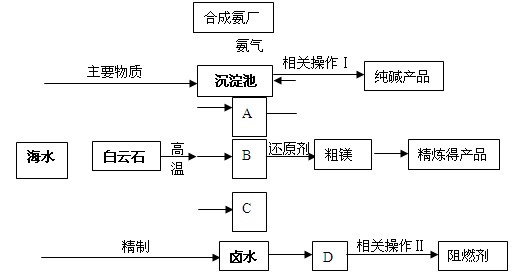

 Һ��(NH4)2SO4��Һ��������Һ���Ƿ���CO32�D�ķ�����_________ ��
Һ��(NH4)2SO4��Һ��������Һ���Ƿ���CO32�D�ķ�����_________ �� ����̿������Ԫ��������þ�������衢ͭ�ȣ�����ȡ�����٣���Pd��99.9%���IJ��ֹ����������£�
����̿������Ԫ��������þ�������衢ͭ�ȣ�����ȡ�����٣���Pd��99.9%���IJ��ֹ����������£�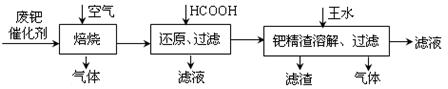
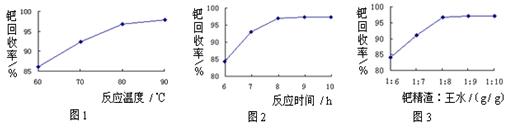
 CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���
CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���
 2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺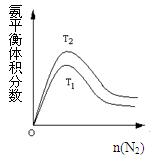
 2(g)+CO(g) ��ƽ�ⳣ��K1
2(g)+CO(g) ��ƽ�ⳣ��K1

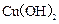 ��ʹ��Һ�ָ���ԭ��Ũ��
��ʹ��Һ�ָ���ԭ��Ũ��