��Ŀ����
X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �� �� �� Ϣ |
| X | X����������ˮ��������̬�⻯������γ�һ���� |
| Y | ���������õİ뵼����ϣ��㷺Ӧ���ڹ����Ϣ���� |
| Z | Z��һ�ֺ���������Ϊ27��������Ϊ14 |
| W | ����������Ӧ��ˮ������һ�ֲ�����ˮ����ɫ���� |
��1��Zλ��Ԫ�����ڱ���__________���ڵ�_________�壻Z��ԭ�Ӱ뾶��Y��________�� X�ĵ�һ�����ܱ�Y��________�����С������
��2��W��̬ԭ�ӵĺ�������Ų�ʽΪ____ ____��XH3���ӵķе�ϸߣ��������ԭ��____ ��
��3��X���⻯�X2H4�����Ʊ�����֮һ�ǽ�NaClO��Һ��XH3��Ӧ�Ƶã���д���÷�Ӧ�����ӷ���ʽ ��
��4����֪�������ݣ�4W(s)+ O2(g) = 2W2O(s) ��H =" -337.2" KJ/mol 2W(s)+ O2(g) = 2WO(s) ��H =" -314.6" KJ/mol����W2O��O2��Ӧ����WO���Ȼ�ѧ����ʽ�� ��
��1���� ����A �� �� �� ��
��2��1s22s22p63s23p63d104s1 ��[Ar]3d104s1 ��NH3���Ӽ���������
��3��ClO��+ 2NH3 �� N2H4 + Cl��+ H2O��
��4��2Cu2O(s)+ O2(g)=4CuO(s) ��H = -292.0KJ��mol-1 ��
����������������Ƚ���Ԫ���ƶϡ�X������������ˮ��������̬�⻯������γ�һ���Σ�˵��X�Ƿǽ���Ԫ�أ�������������ˮ�������ᣬ��̬�⻯���ˮ��Һ�Լ��ԡ���X��NԪ�أ���Ӧ����ΪNH4NO3�����������õİ뵼����ϣ��㷺Ӧ���ڹ����Ϣ�����Ԫ����Si��Y��Si��Z��һ�ֺ���������Ϊ27��������Ϊ14����������Ϊ27-14=13.��AlԪ�ء�W������������Ӧ��ˮ������һ�ֲ�����ˮ����ɫ���壬��W��Cu����1��Al�ĺ�������Ų�Ϊ2��8��3��������Ԫ�����ڱ���λ�ڵ������ڵ�IIIA��Al��Si���ǵ������ڵ�Ԫ�أ�����ԭ������������ԭ�Ӱ뾶��С������ԭ�Ӱ뾶Al>Si����Ԫ�صĺ�������Ų�ʽΪ1s22s22p3,2p������ڰ�������ȶ�״̬��ʧȥ�����ѣ���SiԪ�صĺ�������Ų�ʽΪ1s22s22p63s23p2, 3p��������ȶ���״̬���Ƚ�����ʧȥ���ӣ�����ʧȥ���ӽ����ס�X�ĵ�һ�����ܱ�Y�Ĵ�2��29��Ԫ��Cu��̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1 ��[Ar]3d104s1��NH3���ɷ��ӹ��ɵķ��Ӿ��壬�ڷ���֮����˴���һ��ķ��Ӽ����������������������˷���֮�������ã����Կ˷����Ӽ�������ʹ�����ۻ���������Ҫ�������ϸߡ����۵㡢�е�ϸߡ���3��ʹNaClO��Һ��NH3��Ӧ��N2H4�Ļ�ѧ����ʽΪ��NaClO + 2NH3 �� N2H4 + NaCl+ H2O������д�����ӷ���ʽΪClO�� + 2NH3 �� N2H4 + Cl�� + H2O����4���ڡ�2-�ٿɵã�2Cu2O(s)+ O2(g)=4CuO(s) ��H =" -292.0KJ/mol" ��
���㣺����Ԫ�ص��ƶϡ�Ԫ�����ڱ���Ԫ�������ɡ�ԭ�ӽṹ������ṹ�����ӷ���ʽ���Ȼ�ѧ����ʽ����д��֪ʶ��

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�������ѧ��ѧ����Ԫ��ԭ�ӽṹ�����������ʾ��
| ��� | Ԫ�� | �ṹ������ |
| �� | A | A�����������г������������������Ȼ����Է����������35.5 |
| �� | B | Bԭ���������������ڲ���������� |
| �� | C | C�dz������ʵ���ҪԪ�أ����ʳ����³���̬ |
| �� | D | D���ʱ���Ϊ����Ϣ�����Ĵ��������dz��õİ뵼����� |
| �� | E | ͨ������£�Eû�������ϼۣ�A��B��C��D��F������E�γɻ����� |
| �� | F | F�����ڱ��п������ڢ�A�壬Ҳ����������ڢ�A�� |
��1��Aԭ�������ڱ��е�λ��Ϊ________��
��2��B��C�γɵĻ�����Ļ�ѧʽΪ________��������________���������ӡ����ۡ�����
��3��F��E�����γ�10���Ӻ�18���ӵ����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����_______________________________________��
��4��C��E���ǽϻ��õķǽ���Ԫ�أ��û�ѧ����ʽ���������ֵ��ʵ�������ǿ��__________________________________________________________��
��5���о�һ��ʵ����ʵ֤��A��B����Ԫ�ص��ʻ�ԭ�Ե�ǿ��_______________________________________________________________��
������������Ԫ�صĵ�һ�����ĵ���������(��λ�� kJ��mol��1)���ش����и��⣺
| Ԫ�ش��� | I1 | I2 | I3 | I4 |
| Q | 2 080 | 4 000 | 6 100 | 9 400 |
| R | 496 | 4 562 | 6 912 | 9 543 |
| S | 738 | 1 451 | 7 733 | 10 540 |
| T | 578 | 1 817 | 2 745 | 11 575 |
| U | 420 | 3 100 | 4 400 | 5 900 |
��1�������ڱ��У�����ܴ���ͬһ�����________��
A��Q��R B��S��T C��T��U D��R��T E��R��U
��2���������ӵ���������������________��
A��S2�� B��R2�� C��T3�� D��U��
��3������Ԫ���У���ѧ���ʺ�������������QԪ�ص���____��
A���� B���� C���� D����
��4��ÿ��Ԫ�ض������������������ܵ��������ϴ���������һ��ʵ��һ������˵����______�����UԪ���Ƕ�����Ԫ�أ���������ĵ�2�ε����ܷ�Ծ���ݽ�������ʧȥ��_____������ʱ��
��5�����R��S��T��ͬ���ڵ���������Ԫ�أ������ǵ�ԭ��������С�����˳����________������Ԫ��________�ĵ�һ�������쳣�ߵ�ԭ����__________________��
X��Y��Z��M��Q��G���ֶ�����Ԫ�أ�ԭ��������������X��Zͬ���壬���γ����ӻ�����ZX��Y��Mͬ���壬Y��һ��ͬλ��ԭ�ӳ����ڲⶨ����������Q�γɵĵ���Ϊ����ɫ���塣��ش��������⣨�漰���ʾ��û�ѧʽ��ʾ����
��1�����ӻ�����ZX��X���ӵĽṹʾ��ͼΪ �� Y��Ԫ�����ڱ��е�λ����_______________��
��2������Ԫ�ص�����������Ӧ��ˮ����������ǿ����_______________��Q��G����̬�⻯�ﻹԭ�Ը�ǿ����__________________��
��3����ҵ���Ʊ�M�ĸߴ��ȵ��ʣ�����һ����Ҫ��Ӧ�ǣ���MXG3��X2�ڸ����·�Ӧ���÷�Ӧ���̱��������ˮ��������ΪMXG3��ˮ���ҷ�Ӧ����H2�� �� ������������������ĺ���� ��
��4��X2Q��ȼ����Ϊa kJ·mol-1������X2Qȼ�շ�Ӧ���Ȼ�ѧ����ʽ��ȷ���� ��
| A��2X2Q(g) + O2(g) =" 2Q(s)" + 2X2O(g)��H=" -2a" kJ·mol-1 |
| B��X2Q(g) + 2O2(g) = QO3(g) + X2O(l)��H=" +a" kJ·mol-1 |
| C��2X2Q(g)+ 3O2(g) = 2QO2(g) + 2X2O(l)��H=" -2a" kJ·mol-1 |
| D��X2Q(g) + 2O2(g) = QO3(g) + X2O(l)��H=" -a" kJ·mol-1 |
 Fe + 2ZG �ŵ�ʱ����ص�������ӦʽΪ: �õ�صĵ����Ϊ___________________��
Fe + 2ZG �ŵ�ʱ����ص�������ӦʽΪ: �õ�صĵ����Ϊ___________________��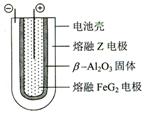
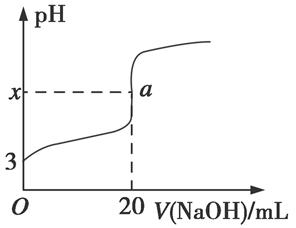
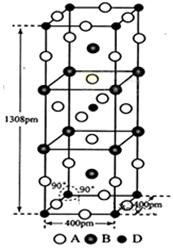
 Si(s)+2CO(g)
Si(s)+2CO(g)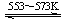 SiHCl3(l)��H2(g) + Q��Q��0��
SiHCl3(l)��H2(g) + Q��Q��0�� Si(��)��3HCl(g)
Si(��)��3HCl(g)