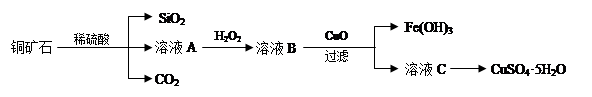��Ŀ����
��һ��(4��)�����е����������ʣ�д����ȥ��Щ���ʵ��Լ���
��1��MgO (Al2O3) ��2��Cl2(HCl)
��3��FeCl3(FeCl2) ��4��NaHCO3��Һ(Na2CO3)
������(6��)��ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ͼ��ʾ��

�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ ��
��������Ҫ��ָ ���Լ��ٿ�ѡ�� ��
��������ָ �������������տɵý���þ��
��������8�֣�ʵ��������480ml 0��1mol��L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� g��
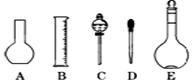
��2����ͼ��ʾ������������Һ�϶�����Ҫ���� (�����)����ʵ�����貣������E���Ϊ mL��
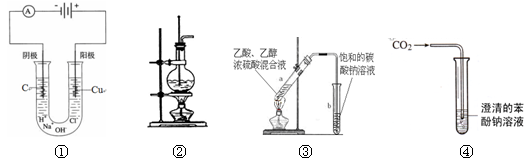
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ���������ַ��ţ�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳���� ������д��ĸ��ÿ������ֻ��ѡ��һ�Σ�
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в����������� ����;������д���֣�
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��
E������ʱ���ӿ̶���
��1��MgO (Al2O3) ��2��Cl2(HCl)
��3��FeCl3(FeCl2) ��4��NaHCO3��Һ(Na2CO3)
������(6��)��ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ͼ��ʾ��

�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ ��
��������Ҫ��ָ ���Լ��ٿ�ѡ�� ��
��������ָ �������������տɵý���þ��
��������8�֣�ʵ��������480ml 0��1mol��L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� g��
��2����ͼ��ʾ������������Һ�϶�����Ҫ���� (�����)����ʵ�����貣������E���Ϊ mL��

��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ���������ַ��ţ�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳���� ������д��ĸ��ÿ������ֻ��ѡ��һ�Σ�
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в����������� ����;������д���֣�
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��
| A������ǰû�н�����ƿ�е�ˮ������ |
| B��̼����ʧȥ�˲��ֽᾧˮ�� |
| C��̼���ƾ��岻�������л����Ȼ��ƣ� |
| D������̼���ƾ���ʱ�����������⣻ |
��һ����4�֣���1�� NaOH��Һ��2������NaCl��Һ��3��Cl2��4��CO2��ÿ��1�֣�
��������6�֣�CaO+H2O+MgCl2=Mg(OH)2+CaCl2��2�֣����ˣ�1�֣� HCl��1�֣�
����Ũ������ȴ�ᾧ�����ˣ�2�֣�
(��)��8�֣���1��14��3����2��AC 500 ��3���٢ۢ�
��4��dbac ��5��2 (6)CE ���ڣ�6����2�֣�����ÿ��1�֣���8�֣�
��������6�֣�CaO+H2O+MgCl2=Mg(OH)2+CaCl2��2�֣����ˣ�1�֣� HCl��1�֣�
����Ũ������ȴ�ᾧ�����ˣ�2�֣�
(��)��8�֣���1��14��3����2��AC 500 ��3���٢ۢ�
��4��dbac ��5��2 (6)CE ���ڣ�6����2�֣�����ÿ��1�֣���8�֣�
�����������һ����1������Al2O3Ϊ�������������NaOH��Һ�ɳ�ȥMgO�е�Al2O3���ʡ�
��2��Cl2�ڱ���NaCl��Һ�е��ܽ�Ⱥ�С�������ñ���NaCl��Һ�ɳ�ȥCl2�е�HCl��
��3��Cl2�ɰ�FeCl2����ΪFeCl3������ͨ��Cl2�ɳ�ȥFeCl2��
��4��CO2��H2O��Na2CO3��Ӧ����NaHCO3������CO2�ɳ�ȥNaHCO3�е�Na2CO3���ʡ�
����������CaO��H2O��Ӧ����Ca(OH)2��CaO+H2O=Ca(OH)2��Ȼ��Ca(OH)2��MgCl2��Ӧ����Mg(OH)2��Ca(OH)2+MgCl2=Mg(OH)2+CaCl2���ӺͿɵ��ܷ���ʽ��CaO+H2O+MgCl2=Mg(OH)2+CaCl2�������ٰ�Mg(OH)2�������������Ϊ���ˣ��Լ�����Mg(OH)2��Ӧ����MgCl2��ΪHCl���������Ǵ�MgCl2��Һ�еõ�MgCl2��6H2O��Ϊ����Ũ������ȴ�ᾧ�����ˡ�
��������1������480ml��Ҫѡ��500ml����ƿ��m(Na2CO3��10H2O)=0��5L��0��1mol/L��286g/mol=14��3g��
��2������һ�����ʵ���Ũ�ȵ���Һ���ò�����ƿ����Һ©������������ƿ�Ĺ��ʵ����������ƿΪ500ml��
��3������ƿ�ϱ����¶ȡ������Ϳ̶��ߣ��ʴ�Ϊ�٢ۢݡ�
��4��������������ƽ�������ʣ�Ȼ������ʷ����ձ��У���ˮ�ܽ⣬����Һע������ƿ������ý�ͷ�ιܶ��ݣ������ڲ���������ʹ�õ�ǰ��˳����dbac��
��5���ܽ�����ʱ�ò��������裬ת����Һʱ�ò��������������Բ�����������2����;��
��6��A�� ����ǰû�н�����ƿ�е�ˮ��������Ũ����Ӱ�죻B��̼����ʧȥ�˲��ֽᾧˮ��Na2CO3���Ũ��ƫ�ߣ�C��̼���ƾ��岻�������л����Ȼ��ƣ�������Na2CO3���٣�Ũ��ƫ�ͣ�D������̼���ƾ���ʱ�����������⣬������Na2CO3�������Ũ��ƫ�ߣ�E������ʱ���ӿ̶��ߣ�ʹ��Һ��������Ũ��ƫ�͡�

��ϰ��ϵ�д�
�����Ŀ