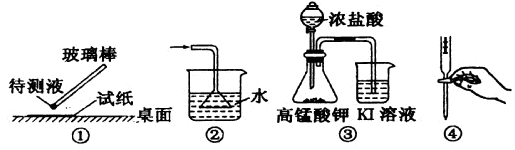
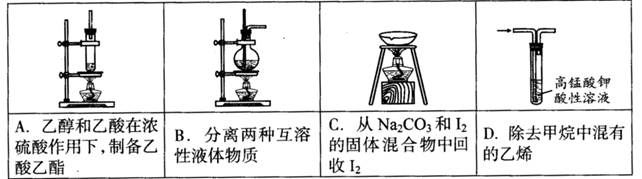
��Ŀ����
ijͭ��ʯ��Ҫ��Cu2(OH)2CO3����������Fe��Si�Ļ����ʵ�����Դ�ͭ��ʯΪԭ���Ʊ�CuSO4��5H2O��CaCO3�����ֲ������£�
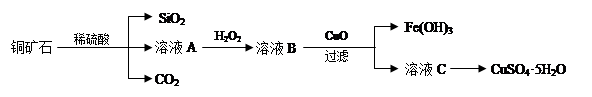
��ش��������⣺
��1����ҺA������Cu2+�⣬�����ܺ��еĽ���������________(�����ӷ���)������ҺA�м���H2O2��Ӧ�����ӷ���ʽ��______________��
��2���������ɵ�CO2��ȡ����̼��ơ��Ʊ�ʱ�������Ȼ�����Һ��ͨ�백������ͨ��CO2��
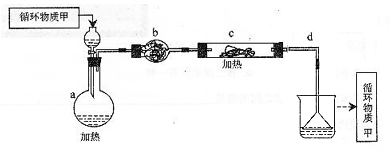
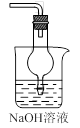
��ʵ����ͨ�����ü����Ȼ�狀��������ƻ����ķ�����ȡ������ijѧϰС��ѡȡ��ͼ��������װ����ȡ���ռ������İ�����

����������������Ӹ������ӿڣ�����Ϊ��ȷ��˳��Ϊa��______��______��______��______�� i��������i����©����������______________��
��ʵ�����л����ù����������ƺ�Ũ��ˮ��ȡ�����������������ʺ���ɸ�ʵ��ļ���װ����
_________(����)

��3���ⶨͭ��ʯ��Cu2(OH)2CO3�����ٷֺ����ķ����ǣ�a����1.25gͭ��ʯ��ȡ��CuSO4��5H2O����ƿ�У���������ˮ��ȫ�ܽ⣻b������Һ�м���100mL0.25mol/L������������ҺʹCu2+��ȫ������c�����ˣ�d����Һ�е�����������Һ��0.5mol/L����ζ����յ㣬����10mL���ᡣ��ͭ��ʯ��Cu2(OH)2CO3��������Ϊ_____________��
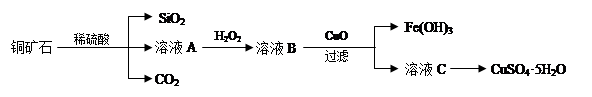
��ش��������⣺
��1����ҺA������Cu2+�⣬�����ܺ��еĽ���������________(�����ӷ���)������ҺA�м���H2O2��Ӧ�����ӷ���ʽ��______________��
��2���������ɵ�CO2��ȡ����̼��ơ��Ʊ�ʱ�������Ȼ�����Һ��ͨ�백������ͨ��CO2��
��ʵ����ͨ�����ü����Ȼ�狀��������ƻ����ķ�����ȡ������ijѧϰС��ѡȡ��ͼ��������װ����ȡ���ռ������İ�����

����������������Ӹ������ӿڣ�����Ϊ��ȷ��˳��Ϊa��______��______��______��______�� i��������i����©����������______________��
��ʵ�����л����ù����������ƺ�Ũ��ˮ��ȡ�����������������ʺ���ɸ�ʵ��ļ���װ����
_________(����)

��3���ⶨͭ��ʯ��Cu2(OH)2CO3�����ٷֺ����ķ����ǣ�a����1.25gͭ��ʯ��ȡ��CuSO4��5H2O����ƿ�У���������ˮ��ȫ�ܽ⣻b������Һ�м���100mL0.25mol/L������������ҺʹCu2+��ȫ������c�����ˣ�d����Һ�е�����������Һ��0.5mol/L����ζ����յ㣬����10mL���ᡣ��ͭ��ʯ��Cu2(OH)2CO3��������Ϊ_____________��
��1��Fe2+��Fe3+��2�֣���2Fe2++H2O2 +2H+��2Fe3++2H2O ��2�֣�
��2����a��g��h��e��d�� i ��2�֣�����ֹ������1�֣�����A��2�֣� ��3��88.8%��2�֣�
��2����a��g��h��e��d�� i ��2�֣�����ֹ������1�֣�����A��2�֣� ��3��88.8%��2�֣�
�����������1��Cu2(OH)2CO3�Լ�Fe��Si�Ļ�������ϡ���ᷴӦ��������ͭ������������������������������ϡ�����Ӧ��������Һ��A�г�����Cu2+�⣬�����ܺ��еĽ���������Fe2+��Fe3+��˫��ˮ����ǿ�����ԣ��ܰ����������������������ӣ���������ҺA�м���H2O2��Ӧ�����ӷ���ʽ��2Fe2++H2O2 +2H+��2Fe3++2H2O��
��2���ٸ���װ��ͼ��֪��Aװ�����Ʊ������ġ��������ɵİ����к���ˮ������������Ҫ���ѡ�ü�ʯ�Ҹ���������ܶ�С�ڿ����ģ��Ұ�����������ˮ������Ӧ���������ſ������ռ�������Ҫ������İ����������գ������ȷ�IJ���˳����a��g��h��e��d�� i��������������ˮ�������i������©���������Ƿ�ֹ������
���ù����������ƺ�Ũ��ˮ��ȡ���������������Ҫ��Һ©������Ӧ����Ҫ���ȣ��Ұ����������ſ������ռ���������ȷ�Ĵ�ѡA��
��3��������������ʵ�����0.01L��0.5mol/L��0.005mol������ݷ���ʽNaOH��HCl��NaCl��H2O��֪�������ᷴӦ������������0.005mol���������Ƶ����ʵ�����0.1L��0.25mol/L��0.025mol����������ͭ��Ӧ������������0.025mol��0.005mol��0.020mol������ݷ���ʽ2NaOH��CuSO4��Cu(OH)2����Na2SO4��֪������ͭ�����ʵ�����0.020mol��2��0.010mol�����Ը���ԭ���غ��֪��ͭ��ʯ��Cu2(OH)2CO3�����ʵ�����0.010mol��2��0.005mol������Cu2(OH)2CO3��������Ϊ
 ��100%��88.8%��
��100%��88.8%��
��ϰ��ϵ�д�
�����Ŀ



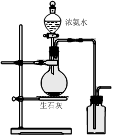


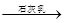 Mg��OH��2
Mg��OH��2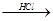 MgCl2��Һ��MgCl2��6H2O��MgCl2
MgCl2��Һ��MgCl2��6H2O��MgCl2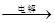 Mg
Mg