��Ŀ����
�����Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�

��ش��������⣺
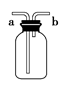
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���Ǣ�_________����__________����________��
�����ܡ����õ�����Ҫ�����ǣ���__________����__________(ÿ����1-2������)��
(2)�ж���Һ��SO42-�����ѳ�����ȫ�ķ�����_________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�����ȷ��ȡһ�����Ŀ�ʯ�������������ܽ⡢Ԥ������
(4)ijͬѧ���÷����������ʯ�е�Fe���������ֲⶨ�������ƫ�ߣ���������Ŀ���ԭ����_____________________________________________��
(5)��ȡ��ʯ����1.60 g, ��������������Ƶ�BaSO4������Ϊ4.66 g�������ʯ�е���Ԫ��ȫ��������FeS2����ÿ�ʯ��FeS2������������___________��

��ش��������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���Ǣ�_________����__________����________��
�����ܡ����õ�����Ҫ�����ǣ���__________����__________(ÿ����1-2������)��
(2)�ж���Һ��SO42-�����ѳ�����ȫ�ķ�����_________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�����ȷ��ȡһ�����Ŀ�ʯ�������������ܽ⡢Ԥ������
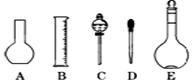
| A���ô��п̶ȵ��ձ����Ƴ�100 mL������Һ�� | B������Ͳ��ȡ25.00 mL������Һ�� | C����������ƿ�С� | D��������ˮϴ�ӵζ��ܺ�װ��KMnO4����Һ���øñ���Һ�ζ�����������(E)����Һ��ɵ��Ϻ�ɫʱ��ֹͣ�ζ�����30���ڲ���ɫ��(F)��ȡ������ζ��������ĵ�KMnO4����Һ��������������е�FeԪ�غ�������ָ����ʵ������д����������ı��________________________�� |
(5)��ȡ��ʯ����1.60 g, ��������������Ƶ�BaSO4������Ϊ4.66 g�������ʯ�е���Ԫ��ȫ��������FeS2����ÿ�ʯ��FeS2������������___________��
��1������ ��1�֣� ϴ�� ��1�֣� ���� ��1�֣� �������ƾ��� ��1�֣� ��ƽ ��1�֣�
��2��ȡ�ϲ���Һ�μ�BaCl2��Һ�����ް�ɫ�������ɣ�˵��SO42-������ȫ ��2�֣�
��3��A��B��D ��3�֣�
��4����2�֣����������ܵ�ԭ��д������һ�֣�����2�֣�
�� Fe(OH)3���������������������
�ڹ���ϴ��ʱδ��ֽ�����������ϴȥ
�� Fe(OH)3���ղ���֣�δ��ȫת��ΪFe2O3
��5��75.0% ��3�֣�
�����������1������Һ�еõ������Ĺ����辭�����ˡ�ϴ�ӡ������������ڢܲ�Ϊ����ʹ���������ֽ�Ϊ��������Ȼ�����������������ȷ��FeS2����������ʹ�õ������ֱ�Ϊ�������ƾ��ƺ���ƽ����2��ȡ�ϲ���Һ�μ�BaCl2��Һ�����ް�ɫ�������ɣ�˵��SO42-������ȫ����3��A��������ҺҪ������ƿ������B����Ͳ�Ķ���ֻ�ܾ�ȷ��ʮ��λ������D���ζ���Ҫ�ô�װҺ��ϴ������4�������Ĺ��������ߣ����½��ƫ�ߣ��ʿ��ܵ�ԭ���У��� Fe(OH)3��������������������� �ڹ���ϴ��ʱδ��ֽ�����������ϴȥ �� Fe(OH)3���ղ���֣�δ��ȫת��ΪFe2O3��
��5��n(FeS2)=n(SO42-)/2=2n(BaSO4)/2=4.66��233��2=0.01mol
�ÿ�ʯ��FeS2������������0.01��120��1.60=0.75

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ