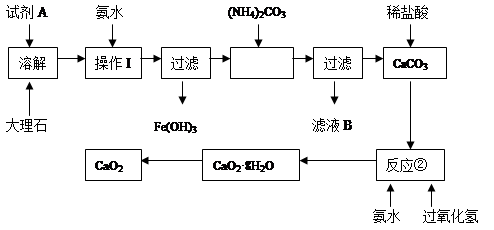
��Ŀ����
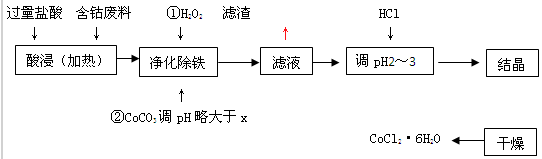
�й��ܺ�������������ʼ��±���
�ú��ܷ��ϣ��������������Ʊ��Ȼ��ܣ�Co+2HCl=CoCl2+H2�������������£�
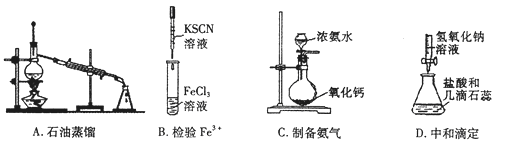
�Իش�
��1���������������У�д������CoCO3�����ܣ���pHʱ��Ӧ�����ӷ���ʽ
��2����Һ�У�Fe3+����1.0��10-5mol/Lʱ������ΪFe3+������ȫ���Լ��������£�Fe(OH) 3������ȫʱ����x= ��
��3���ڲ�����CoCO3��pH�Դ���x��ԭ���� ��
��4����Һ������������ �� ��
��5��Ϊ�˷�ֹCoCl2��6H2O��ˮ�������ʱ�˲��õķ���������� �� ��
| ��ѧʽ | �ܶȻ�������ʱ��Ksp | ������ȫʱ��pH | �Ȼ��ܾ�������� |
| Co(OH) 2 | 5.9��10-15 | 9.4 | CoCl2��6H2O�ʺ�ɫ���������ȶ���110ºC��120ºCʱ��ˮ�����ɫ��ˮ�Ȼ��� |
| Fe(OH) 2 | 1.6��10-14 | 9.6 | |
| Fe(OH) 3 | 1.0��10-35 | x |
�ú��ܷ��ϣ��������������Ʊ��Ȼ��ܣ�Co+2HCl=CoCl2+H2�������������£�

�Իش�
��1���������������У�д������CoCO3�����ܣ���pHʱ��Ӧ�����ӷ���ʽ
��2����Һ�У�Fe3+����1.0��10-5mol/Lʱ������ΪFe3+������ȫ���Լ��������£�Fe(OH) 3������ȫʱ����x= ��
��3���ڲ�����CoCO3��pH�Դ���x��ԭ���� ��
��4����Һ������������ �� ��
��5��Ϊ�˷�ֹCoCl2��6H2O��ˮ�������ʱ�˲��õķ���������� �� ��
��1��CoCO3+2H+=Co2++H2O+CO2��
��2��4
��3��ʹFe3+��ȫ����
��4��CoCl2
��5����ѹ���¶ȵ���110�����HCl�����м��ȣ���д2�㣩
��2��4
��3��ʹFe3+��ȫ����
��4��CoCl2
��5����ѹ���¶ȵ���110�����HCl�����м��ȣ���д2�㣩
�����������1���������������У�����CoCO3�����ܣ���CoCO3����̼���Σ������ᷴӦ��CoCO3+2H��=Co2��+H2O+CO2����ͨ���˷�Ӧ������Һ�����ԣ�����Һ��pH����Ϊ��CoCO3+2H��=Co2��+H2O+CO2����
��2����Һ�У�Fe3������1.0��10-5mol��L��1ʱ������ΪFe3��������ȫ��Ksp[Fe(OH)3 ]=1.0��10-35=c��Fe3������c3��OH����=1.0��10-5mol��L-1��c3��OH����������c��OH����=10-10mol��L-1��c��H����=
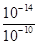 mol��L-1=10-4mol��L-1��PH=-lgc��H����=-lg10-4=4������4��
mol��L-1=10-4mol��L-1��PH=-lgc��H����=-lg10-4=4����Ϊ��4����3�����ݣ�2��������֪��Ϊ��ʹFe3����ȫ������pHӦ�Դ���4����Ϊ��ʹFe3����ȫ������
��4����ȥFe3������Һ����Ҫ����NaCl��CoCl2���Լ���������HCl����Ϊ��CoCl2��NaCl��
��5����CoCl2��6H2O��110��C��120��Cʱ��ˮ�����ɫ��ˮ�Ȼ��ܣ����Բ����ø��º�ɣ�Ϊ�˷�ֹCoCl2��6H2O��ˮ�������ʱ�˲��õķ���������Ǽ�ѹ���¶ȵ���110�����HCl�����м��ȣ���Ϊ����ѹ���¶ȵ���110�棻����HCl�����м��ȣ�

��ϰ��ϵ�д�
�����Ŀ