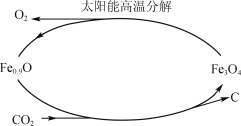
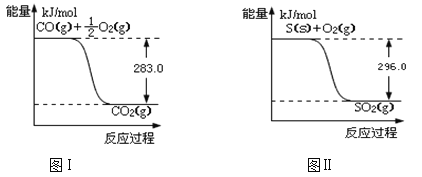
��Ŀ����
����Ŀ���⻯�(LiH)�ڸ���Ŀ��������ȶ����ڣ���ˮ�����ܹ�����ȼ�ա�ij�С����ʹ������װ���Ʊ�LiH���塣
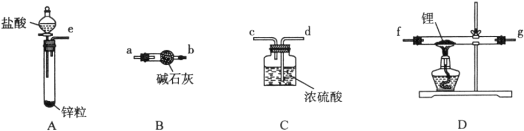
��ͬѧ��ʵ�鷽�����£�
��1����������װ���ӣ���������װ�ýӿڵ�����˳��Ϊ____________________������ҩƷǰ����Ҫ���е�ʵ�������____________________(����д������IJ�������)������װ��B��������________________��
��2������ҩƷ�������Ӵ��Լ�ƿ��ȡ��һ���������(����ʯ���ܷ�)��Ȼ���ڼױ��н�ϴ���Σ��ò�����Ŀ����________________________________________��Ȼ����ٰ�﮷��뵽ʯӢ���С�
��3��ͨ��һ��ʱ�����������ʯӢ�ܣ��ڼ���D����ʯӢ��֮ǰ��������е�ʵ�������______________________________________________________________________��
��4������һ��ʱ���ֹͣ���ȣ�����ͨ������ȴ��Ȼ��ȡ��LiH��װ�뵪���ƿ������ڰ�������ȡ����������Ŀ����Ϊ�˱���LiH������е�ˮ�����Ӵ�������Σ�ա�(��Ӧ����ʽ��
LiH + H2O = LiOH + H2��)�������÷�Ӧԭ�������LiH����ˮ�Ҵ���Ӧ�Ļ�ѧ����ʽ_________________ ___________________��
��5��ȷ�����ƵõIJ�Ʒ0.174g����һ��������������ˮ��Ӧ���ռ�������0.021mol�����Ʒ��LiH��Li�����ʵ���֮��Ϊ____________________��
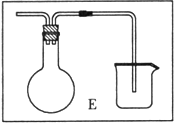
��6����ͬѧ�Լ�ʵ�鷽��������ɣ�����Ϊδ��Ӧ��H2����ֱ���ŷţ����������������װ��E�����ռ�H2���뽫Eװ�ò���������
���𰸡���1��eabfgdc (f��g����Ҳ����)�� ����װ�õ������� ��ȥH2�е�H2O��HCl
��2����ȥﮱ����ʯ����3���ռ�c���ų������岢����H2���� (ֻд����H2����Ҳ����)
��4��LiH + CH3CH2OH ![]() CH3CH2OLi + H2����5��10��1
CH3CH2OLi + H2����5��10��1
��6��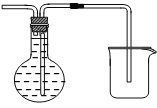
��������
�����������1��������﮷�����Ӧ����LiH���Ʊ�LiH������Ҫ�Ʊ�������LiH�ڸ���Ŀ��������ȶ����ڣ���ˮ�����ܹ�����ȼ�գ������Ʊ��õ�������������﴿��������Aװ���Ʊ���������װ��B�еļ�ʯ�ҳ�ȥ�����е��Ȼ����ˮ������ͨ��װ��D�м��Ⱥ�﮷�Ӧ�����⻯ﮣ��������װ��C����ֹ�����е�ˮ�����Ͷ�����̼����װ��D�����ɵ��⻯﮷�����Ӧ��װ������˳��Ϊ��e��a��b��f��g��d���Ʊ�����װ�ã�����ҩƷǰ����Ҫ���е�ʵ������ǣ�����װ�������ԣ�װ��B�м�ʯ���������ƺ��������ƵĻ�����������ˮ�ɷ�Ӧ���������ƿ�������������������ܷ�Ӧ�����Ը�װ�õ�����������ˮ�����Ͳ����Ȼ������壬�ʴ�Ϊ��e��a��b��f��g��d������װ�������ԣ���ȥH2�е�H2O��HCl��
��2��ȡ��һ���������(����ʯ���ܷ�)��Ȼ���ڼױ��н�ϴ���Σ�����ʯ�����л������ܽ����л��ܼ��ױ��У�������Ŀ���dz�ȥﮱ����ʯ�����ʴ�Ϊ����ȥﮱ����ʯ����
��3��ʹ��ǰӦͨ��һ��ʱ���������ž�װ���ڵĿ�������ֹ����ʱ��������������ըΣ�գ��ڼ���D����ʯӢ��֮ǰ��������е�ʵ������ǣ��ռ�c���ų������岢����H2���ȣ��ʴ�Ϊ���ռ�c���ų������岢����H2���ȣ�
��4��LiH��H2O����ˮ�ⷴӦ������ӽ�����������ӣ��⸺�����������ӽṹ����������LiH���Ҵ���Ӧ���ƣ��ǻ��ṩHԭ����LiH��Ӧ�������������ⲿ�ֽ������CH3CH2OLi����Ӧ����ʽΪ��LiH+CH3CH2OH=CH3CH2OLi+H2�����ʴ�Ϊ��LiH+CH3CH2OH=CH3CH2OLi+H2����
��5����LiH��Li�����ʵ����ֱ�Ϊxmol��ymol����
LiH+H2O=H2��+LiOH
xmol xmol
2Li+2H2O=2LiOH+H2��
ymol 0.5y mol
��8x+7y��0.174��22.4(x+0.5y)��0.4704��
���x=0.02��y=0.002
��LiH��Li�����ʵ���֮��Ϊ0.02mol:0.002mol=10:1���ʴ�Ϊ��10:1��
��6��װ��E�����ռ�H2�����õ�������ƿ���ռ����������Ը�����ˮ���������װ�ã������ܶ̽�������װ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�