��Ŀ����
Ϊ�ⶨ��������茶��塾��NH4��2Fe��SO4��2?xH2O�������ĺ�����ijʵ��С����������ʵ�飺����һ���õ�����ƽȷ����5.000g��������茶��壬���Ƴ�250ml��Һ��
�������ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ����0.010mol/L KMnO4��Һ�ζ���Fe2+ǡ��ȫ��������Fe3+��ͬʱ��MnO4-����ԭ��Mn2+��
���ظ���������Σ�
��ش��������⣺
��1�����������������Һ�IJ������������ǣ�������______��ת�ơ�ϴ�Ӳ�ת�ơ�______��ҡ�ȣ�
��2����______�ζ���ʢ��KMnO4��Һ��
��3�����������һ��KMnO4��Һ������______��������ζ��յ㣮��Ӧ�����ӷ���ʽ��______
��4���ζ�������±���ʾ��
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.05 | 21.04 |
| 2 | 25.00 | 1.50 | 24.50 |
| 3 | 25.00 | 0.20 | 20.21 |
��5�����ݲ������գ�
�ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���KMnO4����Һ���еζ���������Ʒ����������������______�����ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�δ�����ζ�ʱ��ȥKMnO4����Һ�������______���ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�______��
A���ζ�����Һ��ı仯B����ƿ����Һ��ɫ�ı仯
�ܵζ����Ӷ����������Ʒ����������������______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��2��KMnO4��ҺӦ����ʽ�ζ���ʢװ��
��3��Fe2+��KMnO4����������ԭ��Ӧ���ζ��յ�ʱ����Һ�ɻ�ɫ����Ϻ�ɫ���Ұ�����ڲ���ɫ��
��4�����������ƽ��ֵ�����ݷ�Ӧ�����ӷ���ʽ���㣻
��5���ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���KMnO4����Һ���еζ����ζ���û����ϴ��Ũ��ƫ�ͣ����ƫ��
����ƿ�Ƿ����Եζ����û��Ӱ�죻
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
�ܵζ����Ӷ������ᵼ�����ƫС��
����⣺��1��������Һ��Ҫ�������ܽ⡢ת�ơ�ϴ�Ӳ�ת�ơ����ݡ�ҡ�ȵȲ������ʴ�Ϊ���ܽ⣻���ݣ�
��2��KMnO4��Һ����ǿ�����ԣ�Ӧ����ʽ�ζ���ʢװ�ʴ�Ϊ����ʽ��
��3��Fe2+��KMnO4����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪMnO4-+5Fe2++8 H+�TMn2++5Fe3++4H2O��
�ζ��յ�ʱ����Һ�ɻ�ɫ����Ϻ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����Һ�ɻ�ɫ����Ϻ�ɫ���Ұ�����ڲ���ɫ��MnO4-+5Fe2++8 H+�TMn2++5Fe3++4H2O��
��4��ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ����0.010mol/L KMnO4��Һ�ζ������ø��������Һ���Ϊ
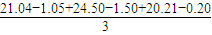 mL=21mL��
mL=21mL��n��MnO4-��=0.021L×0.01mol/L=2.1×10-4mol��
��n��Fe2+��=5×2.1×10-4mol=1.05×10-3mol��
5.000g��������茶��庬��n��Fe2+��=1.05×10-2mol��
n��Fe2+��=56g/mol×1.05×10-2mol=0.588g��
ʵ���øþ�����������������Ϊ
 ��11.20%��
��11.20%���ʴ�Ϊ��11.20%��
��5�����ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���KMnO4����Һ���еζ����ζ���û����ϴ��Ũ��ƫ�ͣ����ƫ�ⶨ�ṹƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
����ƿ�Ƿ����Եζ����û��Ӱ�죬�ʴ�Ϊ����Ӱ�죻
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯���ʴ�Ϊ��B��
�ܵζ����Ӷ������ᵼ�����ƫС���ʴ�Ϊ��ƫ�ͣ�
���������⿼�����ʵĺ����ⶨ�������ڵζ������Ŀ��飬��Ŀ�ѶȲ���ע��������ʵ�����������ע�����

 ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�Ϊ�ⶨ��������茶��塾��NH4��2Fe (SO4)2 �� xH2O�������ĺ�����ijʵ��С����������ʵ�飺
����һ���õ�����ƽȷ����5.000g��������茶��壬���Ƴ�250ml��Һ��
�������ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ����0.010mol/L KMnO4��Һ�ζ���Fe2+ǡ��ȫ��������Fe3+��ͬʱ��MnO4-����ԭ��Mn2+��
���ظ���������Ρ�
��ش��������⣺
��1�����������������Һ�IJ������������ǣ������� ��ת�ơ�ϴ�Ӳ�ת�ơ� ��ҡ�ȡ�
��2���� �ζ���ʢ��KMnO4��Һ��
��3�����������һ��KMnO4��Һ,���� ,������ζ��յ㡣��Ӧ�����ӷ���ʽ��
��4���ζ�������±���ʾ��
|
����� |
������Һ�����/mL |
����Һ����� |
|
|
�ζ�ǰ�̶�/mL |
�ζ���̶�/mL |
||
|
1 |
25.00 |
1.05 |
21.04 |
|
2 |
25.00 |
1.50 |
24.50 |
|
3 |
25.00 |
0.20 |
20.21 |
ʵ���øþ�����������������Ϊ ����������λС����
��5�����ݲ������գ�
�ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���KMnO4����Һ���еζ���������Ʒ���������������� �����ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�δ�����ζ�ʱ��ȥKMnO4����Һ�������
���ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲� ��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ܵζ����Ӷ����������Ʒ���������������� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
Ϊ�ⶨ��������茶��塾��NH4��2Fe��SO4��2?xH2O�������ĺ�����ijʵ��С����������ʵ�飺
����һ���õ�����ƽȷ����5.000g��������茶��壬���Ƴ�250ml��Һ��
�������ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ����0.010mol/L KMnO4��Һ�ζ���Fe2+ǡ��ȫ��������Fe3+��ͬʱ��MnO4-����ԭ��Mn2+��
���ظ���������Σ�
��ش��������⣺
��1�����������������Һ�IJ������������ǣ�������______��ת�ơ�ϴ�Ӳ�ת�ơ�______��ҡ�ȣ�
��2����______�ζ���ʢ��KMnO4��Һ��
��3�����������һ��KMnO4��Һ������______��������ζ��յ㣮��Ӧ�����ӷ���ʽ��______
��4���ζ�������±���ʾ��
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.05 | 21.04 |
| 2 | 25.00 | 1.50 | 24.50 |
| 3 | 25.00 | 0.20 | 20.21 |
��5�����ݲ������գ�
�ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���KMnO4����Һ���еζ���������Ʒ����������������______�����ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�δ�����ζ�ʱ��ȥKMnO4����Һ�������______���ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�______��
A���ζ�����Һ��ı仯B����ƿ����Һ��ɫ�ı仯
�ܵζ����Ӷ����������Ʒ����������������______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����