��Ŀ����
��2012?�Ͼ�ģ�⣩ʵ����������李�����ͷ���м�Ʊ���������茶���ķ������£�
����1��������м�������ȵ�̼������Һ������ˮϴ�ӣ�
����2����ʢ�нྻ��м���ձ��м���ϡH2SO4��Һ��ˮԡ���ȣ�ʹ��м��ϡ���ᷴӦ����������ð������Ϊֹ�����ȹ��ˣ�
����3������Һ�м���һ�����ģ�NH4��2SO4���壮
��1 �����������塢����李���������茶�����ܽ�ȣ�g/100g H2O��
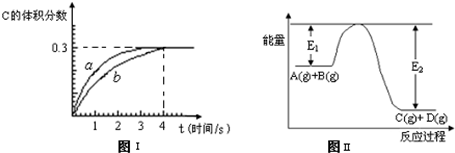
��1������2�п������ɵ����������в����������ķ�����
��2����0��60�淶Χ�ڣ�����������������淋Ļ����Һ�пɻ����������茶����ԭ����
��3��Ϊ�˴Ӳ���3������Һ�л����������茶��壬������
��4���ⶨ��������茶�����Fe2+�����IJ������£�
����1��ȷ��ȡ��������茶�����Ʒa g��ԼΪ0.5g���������Ƴ�100mL��Һ��
����2��ȷ��ȡ25.0mL�����������Һ��250mL��ƿ�У�
����3��������Ũ��ԼΪ0.1mol?L-1KMnO4��Һ�ζ�����Һ���ȶ��ķۺ�ɫ����Ϊ�յ㣻
����4����ʵ�鲽��1��3�ظ�2�Σ�
�ٲ���1������100mL��Һ��Ҫ�IJ���������
��Ϊ�����Ʒ��Fe2+�ĺ��������貹���ʵ����
����1��������м�������ȵ�̼������Һ������ˮϴ�ӣ�
����2����ʢ�нྻ��м���ձ��м���ϡH2SO4��Һ��ˮԡ���ȣ�ʹ��м��ϡ���ᷴӦ����������ð������Ϊֹ�����ȹ��ˣ�
����3������Һ�м���һ�����ģ�NH4��2SO4���壮
��1 �����������塢����李���������茶�����ܽ�ȣ�g/100g H2O��
| ���� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� |
| FeSO4?7H2O | 15.6 | 20.5 | 26.5 | 32.9 | 40.2 | 48.6 | - |
| ��NH4��2SO4 | 70.6 | 73.0 | 75.4 | 78.0 | 81.0 | - | 88.0 |
| ��NH4��2SO4?FeSO4?6H2O | 12.5 | 17.2 | 21.0 | 28.1 | 33.0 | 40.0 | 44.6 |
����������
����������
����2����0��60�淶Χ�ڣ�����������������淋Ļ����Һ�пɻ����������茶����ԭ����
��0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С
��0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С
����3��Ϊ�˴Ӳ���3������Һ�л����������茶��壬������
����Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�����
����Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�����
����4���ⶨ��������茶�����Fe2+�����IJ������£�
����1��ȷ��ȡ��������茶�����Ʒa g��ԼΪ0.5g���������Ƴ�100mL��Һ��
����2��ȷ��ȡ25.0mL�����������Һ��250mL��ƿ�У�
����3��������Ũ��ԼΪ0.1mol?L-1KMnO4��Һ�ζ�����Һ���ȶ��ķۺ�ɫ����Ϊ�յ㣻
����4����ʵ�鲽��1��3�ظ�2�Σ�
�ٲ���1������100mL��Һ��Ҫ�IJ���������
��������100mL����ƿ����ͷ�ιܡ��ձ�
��������100mL����ƿ����ͷ�ιܡ��ձ�
����Ϊ�����Ʒ��Fe2+�ĺ��������貹���ʵ����
ȷ�ⶨ�ζ������ĵĸ��������Һ�����
ȷ�ⶨ�ζ������ĵĸ��������Һ�����
����������1�����������Ա�֤��Һ�в���������������
��2�����ݱ�1���ݷ�������0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С��
��3����������Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�������Եõ���������茶��壻
��4���ٸ�������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��ʹ��������
��֪���˸��������Һ����������ݸ��������Һ���������ӵķ�Ӧ�����Լ������Ʒ���������ӵĺ�����
��2�����ݱ�1���ݷ�������0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С��
��3����������Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�������Եõ���������茶��壻
��4���ٸ�������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��ʹ��������
��֪���˸��������Һ����������ݸ��������Һ���������ӵķ�Ӧ�����Լ������Ʒ���������ӵĺ�����
����⣺��1�����ܹ������������ӻ�ԭ���������ӣ��������������Ա�֤���������в�����������
�ʴ�Ϊ��������������
��2��������0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С��������������������淋Ļ����Һ�пɻ����������茶��壬
�ʴ�Ϊ����0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С��
��3����������Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�������������Եõ���������茶��壬
�ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ����
��4��������100mL��Һ��Ҫ�IJ��������У���������100mL����ƿ����ͷ�ιܡ��ձ���
�ʴ�Ϊ����������100mL����ƿ����ͷ�ιܡ��ձ���
����Ҫ֪���ζ����ĵĸ��������Һ�������Ȼ����ݷ�Ӧ������������ӵ����ʵ�����
�ʴ�Ϊ��ȷ�ⶨ�ζ������ĸ��������Һ�������
�ʴ�Ϊ��������������
��2��������0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С��������������������淋Ļ����Һ�пɻ����������茶��壬
�ʴ�Ϊ����0��60�淶Χ�ڣ�ͬһ�¶�����������茶�����ܽ����С��
��3����������Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ�������������Եõ���������茶��壬
�ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˡ���ˮϴ�ӣ����
��4��������100mL��Һ��Ҫ�IJ��������У���������100mL����ƿ����ͷ�ιܡ��ձ���
�ʴ�Ϊ����������100mL����ƿ����ͷ�ιܡ��ձ���
����Ҫ֪���ζ����ĵĸ��������Һ�������Ȼ����ݷ�Ӧ������������ӵ����ʵ�����
�ʴ�Ϊ��ȷ�ⶨ�ζ������ĸ��������Һ�������
���������⿼�����Ʊ���������茶��巽����ע���������������Ϣ��������ѧ֪ʶ��ɼ��ɣ������Ѷ��еȣ�

��ϰ��ϵ�д�
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�
�����Ŀ

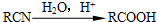
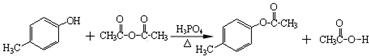
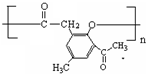
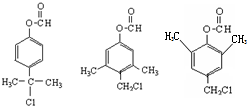
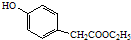 ����һ����Ҫ��ҽҩ�м��壮д����A���Ҵ�Ϊ��Ҫԭ���Ʊ����ǻ������������ĺϳ�·������ͼ�����Լ���ѡ����
����һ����Ҫ��ҽҩ�м��壮д����A���Ҵ�Ϊ��Ҫԭ���Ʊ����ǻ������������ĺϳ�·������ͼ�����Լ���ѡ����