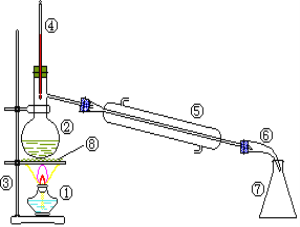
��Ŀ����
����Ŀ����1�����������ǵ���ʵ���___________���Ƿǵ���ʵ���_______���ڱ���ָ��״̬���ܵ������_________�������ϸ��վ�����ţ�
��CO2����Һ̬HCl����ϡ���ᡢ��Al2(SO4)3���塢��NH3��������KOH���ߵ�������
��2���ڱ�״���£����1.32 gij��������Ϊ0.672 L����������Ħ������Ϊ____________��������CO��CO2��������ĵ�Ħ������֮��Ϊ_______________��ͬ��ͬѹ�µ������Ϊ______________��
��3�����ڷ�����ᴿ���ʵij��������У�A����(����) B��ȡ C���� D��Һ��
���и�������ķ�����ᴿӦѡ��������һ�ַ�������ʣ�������ı�ţ�
�ٳ�ȥCa(OH)2��Һ��������CaCO3��_______��
�ڷ������Ȼ�̼��ˮ�Ļ����_______��
�۷������ͺ�ú��_______��
����ȡ��ˮ�еĵⵥ��_______��
���𰸡��ڢܢޢ٢ݢۢޢ�44g/mol7��1111��7CDAB
��������
��1������ˮ��������״̬���ܹ�����Ļ������ǵ���ʣ������ڵ���ʵ���Һ̬�Ȼ��⡢���������塢����KOH������ѡ�ڢܢޣ�����ˮ��������״̬�¾����ܵ���Ļ������Ƿǵ���ʣ������ڷǵ���ʵ��ж�����̼����������ѡ�٢ݣ����������ƶ����ӻ����ӵ����ʿ��Ե��磬���ڱ���ָ��״̬���ܵ������ϡ���ᡢ����KOH������������ѡ�ۢޢߡ�
��2���ڱ�״���£����1.32 gij��������Ϊ0.672 L�����ʵ�����0.672L��22.4L/mol��0.03mol����������Ħ������Ϊ1.32g��0.03mol��44g/mol��������CO��CO2��������ĵ�Ħ������֮��Ϊ28g/mol��44g/mol��7��11��ͬ��ͬѹ�µ�����ȵ������ʵ���֮�ȣ���Ϊ![]() ��
��
��3����̼���������ˮ�����ȥCa(OH)2��Һ��������CaCO3����Ҫ���˳�ȥ����ѡC��
�����Ȼ�̼������ˮ����������Ȼ�̼��ˮ�Ļ������Ҫ��Һ����ѡD��
�����ͺ�ú�ͻ��ܣ��е����ϴ���������ͺ�ú����Ҫ����ѡA��
�ܵ��ʵ��������л��ܼ��У�����ȡ��ˮ�еĵⵥ�ʣ���Ҫ��ȡ����ѡB��

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�����Ŀ���������⡿�״�ˮ������������(SRM)�����������綯���������ӽ���Ĥȼ�ϵ�ص�������Դ,�� ǰ�о���Ҫ��������ߴ������Ժͽ���β����CO����,����ʹȼ�ϵ��Pt�缫�ж����������̷����ķ�Ӧ����:
��ӦI CH3OH(g)+H2O(g) ![]() CO2(g)+3H2(g) ��H1
CO2(g)+3H2(g) ��H1
��Ӧ�� CH3OH(g) ![]() CO(g)+2H2(g) ��H2
CO(g)+2H2(g) ��H2
��Ӧ��.CO(g)+H2O(g) ![]() CO2(g)+H2(g) ��H3
CO2(g)+H2(g) ��H3
���Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2��K3,����K2��K3���¶ȱ仯���±���ʾ:
125�� | 225�� | 325�� | |
K2 | 0.5535 | 185.8 | 9939.5 |
K3 | 1577 | 137.5 | 28.14 |
��ش�:
(1)��Ӧ���ܹ��Է����е�����_______ (�� �����¡��������¡����κ��¶ȡ�), ��H1____��H3 (�� ��>������<���� ��=�� )��
(2)��ͬ������,�״�ˮ������������ϼ״�ֱ�ӷֽ�����(��Ӧ��)���Ƚ�֮������_________��
(3)�ڳ�ѹ��CaO����,CH3OH��H2O�������(�����1��1.2,�����ʵ���2.2mol)���з�Ӧ,tlʱ�̲�� CH3OHת���ʼ�CO��CO2ѡ�������¶ȱ仯����ֱ���ͼ��ʾ(CO��CO2��ѡ����:ת����CH3OH������CO��CO2�İٷֱ�)��
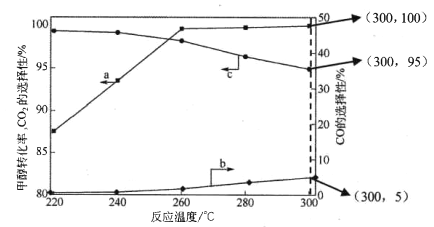
ע:����a��ʾCH3OH��ת����,����b��ʾCO��ѡ����,����c��ʾ CO2��ѡ����
�� ����˵������ȷ����_____��
A.��Ӧ�����¶�Ϊ300��
B.��ҵ����ͨ ���ڸ�ѹ�����½��м״�ˮ��������
C.��֪ CaO�������и��ߴ�����,����״�ƽ��ת����
D.����CaO�ĸ��ϴ����������������
�� 260�� ʱH2���ʵ�����ʱ��ı仯������ͼ��ʾ������300��ʱ��t1ʱ��H2���ʵ�����ʱ��ı仯����_____��
(4)������CO2����������ˮ��Һ�е�����ɼ���,���ɼ���ĵ缫��Ӧʽ��_________��