��Ŀ����
����������Ч����SO2�Կ�������Ⱦ��
(1)��ú�м���ʯ��ʯ�ɼ���ȼ�ղ�����SO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽ��
_______________________________��
(2)��ˮ�������ԣ���Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����HCO3���ȡ���SO2�����������ú�ˮ�����乤��������ͼ��ʾ��
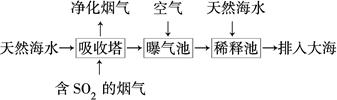
������������ͨ�������Ŀ����_____________________________________��
��ͨ��������������к�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ��������________��
a��Cl�������� b��SO42�������� c��Br�������� d��HCO3��
(3)��NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬�ɵõ�NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ(�缫����Ϊʯī)��
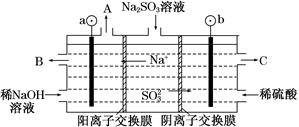
��ͼ��a��Ҫ���ӵ�Դ��________(���������)����C��������������________��
��SO32���ŵ�ĵ缫��ӦʽΪ____________________________��
�۵�����������������������ǿ����ƽ���ƶ���ԭ������ԭ��
__________________________________________��
(1)��ú�м���ʯ��ʯ�ɼ���ȼ�ղ�����SO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽ��
_______________________________��
(2)��ˮ�������ԣ���Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����HCO3���ȡ���SO2�����������ú�ˮ�����乤��������ͼ��ʾ��

������������ͨ�������Ŀ����_____________________________________��
��ͨ��������������к�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ��������________��
a��Cl�������� b��SO42�������� c��Br�������� d��HCO3��
(3)��NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬�ɵõ�NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ(�缫����Ϊʯī)��

��ͼ��a��Ҫ���ӵ�Դ��________(���������)����C��������������________��
��SO32���ŵ�ĵ缫��ӦʽΪ____________________________��
�۵�����������������������ǿ����ƽ���ƶ���ԭ������ԭ��
__________________________________________��
(1)2SO2��O2��2CaCO3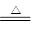 2CaSO4��2CO2
2CaSO4��2CO2
(2)�ٽ�H2SO3��HSO3��������ΪSO42������bd
(3)�ٸ�������
��SO32����2e����H2O=SO42����2H��
��H2O H����OH����������H���ŵ�����H2��c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ
H����OH����������H���ŵ�����H2��c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ
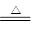 2CaSO4��2CO2
2CaSO4��2CO2(2)�ٽ�H2SO3��HSO3��������ΪSO42������bd
(3)�ٸ�������
��SO32����2e����H2O=SO42����2H��
��H2O
 H����OH����������H���ŵ�����H2��c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ
H����OH����������H���ŵ�����H2��c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ(1)úȼ��ʱ��ʯ��ʯ�ڸ����·ֽ����CaO��CO2��CaOΪ���������������SO2��O2��Ӧ����CaSO4���÷�Ӧ�Ļ�ѧ����ʽ��2SO2��O2��2CaCO3 2CaSO4��2CO2��(2)SO2Ϊ�����������ˮ�������ԣ���������ͨ�������Ŀ���ǽ�SO32����HSO3����������ͨ���������Һ��SO42����Ũ������HCO3����Ũ�ȼ�С��(3)���Na2SO3��Һ������ͼʾ��a��ӦΪ������Ҫ���ӵ�Դ�ĸ�����C�����������������ᡣ�����ĵ缫��ӦʽΪ2H����2e��=H2���������ĵ缫��ӦʽΪSO32����2e����H2O=SO42����2H������������H���������ŵ�����H2������������ƽ��H2O
2CaSO4��2CO2��(2)SO2Ϊ�����������ˮ�������ԣ���������ͨ�������Ŀ���ǽ�SO32����HSO3����������ͨ���������Һ��SO42����Ũ������HCO3����Ũ�ȼ�С��(3)���Na2SO3��Һ������ͼʾ��a��ӦΪ������Ҫ���ӵ�Դ�ĸ�����C�����������������ᡣ�����ĵ缫��ӦʽΪ2H����2e��=H2���������ĵ缫��ӦʽΪSO32����2e����H2O=SO42����2H������������H���������ŵ�����H2������������ƽ��H2O H����OH����c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ��
H����OH����c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ��
 2CaSO4��2CO2��(2)SO2Ϊ�����������ˮ�������ԣ���������ͨ�������Ŀ���ǽ�SO32����HSO3����������ͨ���������Һ��SO42����Ũ������HCO3����Ũ�ȼ�С��(3)���Na2SO3��Һ������ͼʾ��a��ӦΪ������Ҫ���ӵ�Դ�ĸ�����C�����������������ᡣ�����ĵ缫��ӦʽΪ2H����2e��=H2���������ĵ缫��ӦʽΪSO32����2e����H2O=SO42����2H������������H���������ŵ�����H2������������ƽ��H2O
2CaSO4��2CO2��(2)SO2Ϊ�����������ˮ�������ԣ���������ͨ�������Ŀ���ǽ�SO32����HSO3����������ͨ���������Һ��SO42����Ũ������HCO3����Ũ�ȼ�С��(3)���Na2SO3��Һ������ͼʾ��a��ӦΪ������Ҫ���ӵ�Դ�ĸ�����C�����������������ᡣ�����ĵ缫��ӦʽΪ2H����2e��=H2���������ĵ缫��ӦʽΪSO32����2e����H2O=SO42����2H������������H���������ŵ�����H2������������ƽ��H2O H����OH����c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ��
H����OH����c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ��
��ϰ��ϵ�д�
�����Ŀ
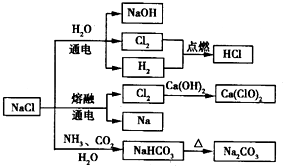
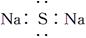
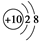
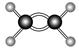
 ��
�� ��
�� ��
�� ��Ϊͬ��������
��Ϊͬ�������� ��NH4Cl�ĵ���ʽΪ
��NH4Cl�ĵ���ʽΪ
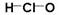

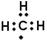
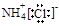
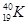

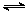 H2CO3+H3O+
H2CO3+H3O+