��Ŀ����
9����1��ԭ�ӽṹ��Ԫ�����ڱ�������������ϵ��������ѧ���ʽṹ֪ʶ���ش��������⣺�پ��У�n-1��d10ns2�����Ų���Ԫ��λ�����ڱ���ds���͢�B�壮
���ճ������й㷺Ӧ�õIJ���֣��������������������˸�Ԫ�أ���Ԫ�ػ�̬ԭ��δ�ɶԵ�����Ϊ6��
��2����CO��Ϊ�ȵ�����ķ��Ӻ����ӷֱ�ΪN2��CN-������һ�ּ��ɣ��ѧʽ��
��3���Ȼ�������
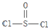 ����һ�ֺ���Ҫ�Ļ�ѧ�Լ���������Ϊ�Ȼ�������ˮ�����Ȼ��������ӵļ��ι����������Σ�����ԭ�Ӳ�ȡSP3�ӻ���ʽ��
����һ�ֺ���Ҫ�Ļ�ѧ�Լ���������Ϊ�Ȼ�������ˮ�����Ȼ��������ӵļ��ι����������Σ�����ԭ�Ӳ�ȡSP3�ӻ���ʽ����4����������Fe[��SCN��]2+�У��ṩ�չ�����ܹ¶Ե��ӵ�����Fe3+��
��5�����־���ͷǾ�����ɿ��Ŀ�ѧ�����ǶԹ������X-��������ʵ�飮
���� ��1���ٸ���Ԫ��ԭ�ӵ���Χ�����Ų����������ɽ�Ԫ�����ڱ��ֳ��������s����p����d����ds����f������n-1��dȫ���������ĵ�����s�����Ϊds��Ԫ�أ�
�ڸ��ݸ�Ԫ�صĻ�̬ԭ�ӵ����Ų�ʽ��������
��2�����ݵȵ�������ԭ������ͬ���۵�������ͬ������
��3�����ݼ۵��ӶԻ�������ȷ�����Ŀռ乹�ͣ�SOCl2��Sԭ�ӳ�2��S-Cl����1��S=O���Ӻ���1�Թ¶Ե��ӣ������ӻ��������4���ݴ˷�����
��4�������������ԭ���ṩ�չ���������ṩ�µ��Ӷԣ�
��5�����־���ͷǾ�����ɿ��Ŀ�ѧ�����ǶԹ������X-��������ʵ�飻
��� �⣺��1����������s���ӣ�Ϊs��Ԫ�أ�������A����A��HeԪ�أ�������p������ӣ�Ϊp��Ԫ�أ���ҪΪ�����0��Ԫ�أ�������d������ӣ�Ϊd��Ԫ�أ�������B������B�͢����壨�ϵ����ϵ���⣩��Ϊ����Ԫ�أ���n-1��dȫ���������ĵ�������s����ϣ�Ϊds��Ԫ�أ�������B����BԪ�أ�Ϊ���ɽ�������n-1��d10ns2���ӹ��͵�Ԫ�أ���n-1��dȫ���������2����������s����ϣ�Ϊds��Ԫ�أ�
�ʴ�Ϊ��ds����B��
�ڸ�Ԫ�صĻ�̬ԭ�ӵ����Ų�ʽΪ��1s22s22p63s23p63d24s44p2����Ԫ�ػ�̬ԭ��δ�ɶԵ�����Ϊ6���ʴ�Ϊ��6��
��2��CO����2��ԭ��14�����ӣ�����CO��Ϊ�ȵ������һ�ַ��Ӻ�һ�����ӵĻ�ѧʽΪ��N2��CN-����O22+��C22-��NO+�����ʴ�Ϊ��N2��CN-��
��3�����ݼ۵��ӶԻ�������ȷ�����Ŀռ乹�ͣ�SOCl2��Sԭ�ӳ�2��S-Cl����1��S=O������1�Թ¶Ե��ӣ������ӻ��������4����Sԭ�Ӳ�ȡSP3�ӻ���������״Ϊ�����Σ��ʴ�Ϊ�������Σ�SP3��
��4�������������[Fe��SCN��]2+�У�����������Fe3+���ṩ�ṩ�չ�����ܹ¶Ե��ӣ�
�ʴ�Ϊ��Fe3+��
��5�����־���ͷǾ�����ɿ��Ŀ�ѧ�����ǶԹ������X-��������ʵ�飬�ʴ�Ϊ��X-��������ʵ�飻
���� ������һ���ṹ��ѧ֪ʶ���ۺ���Ŀ������ѧ�������ͽ��������������ۺ��Խ�ǿ���ѶȽϴ�

| A�� | ������Һ�У�Na+��K+��MnO4-��CO32- | |
| B�� | ������Һ�п��ܴ�������Na+��ClO-��SO42-��I- | |
| C�� | 0.1 mol•L-1 NH4HCO3��Һ�У�K+��Na+��NO3-��Cl- | |
| D�� | 0.1 mol•L-1 FeCl3��Һ�У�Fe2+��NH4+��SCN-��SO42- |
| A�� | ͭ��ϡ���ᷴӦ Cu+2NO3-+4H+=Cu2++2NO2��+2H2O | |
| B�� | ϡ̼������Һ��ͨ�������̼ CO32-+CO2+H2O=2HCO3- | |
| C�� | ���Ȼ�����Һ�е��백ˮ Al3++4NH3•H2O=AlO2-+4NH4+ | |
| D�� | ����������Һ��ͨ��SO2 Ca2++2ClO-+H2O+SO2=CaSO3��+2HClO |
| A�� | SO2��Ũ��Ϊ0.25 mol•L-1��SO3��Ũ��Ϊ0.25mol•L-1 | |
| B�� | SO3��Ũ��Ϊ0.3mol•L-1 | |
| C�� | SO2��Ũ��Ϊ0.4 mol•L-1��O2��Ũ��Ϊ0.2 mol•L-1 | |
| D�� | SO3��Ũ��Ϊ0.4 mol•L-1 |

| A�� | a������b���� | |
| B�� | ����������a�缫�������� | |
| C�� | ���Ӵ�b����a���ƶ� | |
| D�� | ������ӦʽΪ��SO2+2H2O-2e-=SO42-+4H+ |
��1���������۲���H2��˵�������л�ԭ�ԣ�������ԡ���ԭ�ԡ�����
��2����ͬѧ��������H+�������ڣ���NO3-�Ͳ��ܴ������ڣ����ʵ��֤ʵ�����
| װ �� | �� �� |
 | ��ʵ���ʼ��δ���������� ��һ������������ݣ�Һ���Ϸ���dz��ɫ ���Թܱ��ȣ���Һ���� |
�ڸ���������Ʋ���Һ�в�����NO��Ϊ��һ��ȷ�ϣ���������ʵ�飺
| ʵ �� | �� �� | �� �� |
| ʵ��1 | ��ʪ��KI-������ֽ���ڿ����� | δ���� |
| ʵ��2 | ��ʪ��KI-������ֽ����dz��ɫ���� | ��ֽ���� |
b��ʵ��1��Ŀ���ǶԱ�ʵ�飬�ų�����ʹʪ��ĵ���KI��ֽ�����Ŀ��ܣ�
c��ʵ��1��2˵����Ӧ������NO��д���÷�Ӧ�����ӷ���ʽ��Al+NO3?+4H+�TAl3++NO��+2H2O��
��3���ټ��裺��OH-�������ڣ�NO3-Ҳ���ܲ��ܴ������ڣ��������ʵ��֤ʵ�����
| װ�� | ���� |
 | ��ʵ���ʼ��δ���������� ��һ������������ݣ��д̼�����ζ |
�ٴ̼�����ζ��������NH3��
�ڲ�������������ӷ���ʽ��8Al+3NO3-+5OH-+2H2O�T3NH3��+8AlO2-��
��4����NaOH��Һ�м������ۣ����ֻ�������H2���ɣ�ʵ����֤ʵ��NO3?���ᡢ���Ի����ж���һ���������ԣ������������ʣ�������������������ɫ��Һһ���ܴ������ڵ���K+��OH-��
| A�� | CH4+Cl2$\stackrel{����}{��}$CH3Cl+HCl | B�� | CH2=CH2+HCl$\stackrel{����}{��}$CH3CH2Cl | ||
| C�� | 2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O | D�� | 2C6H6+15O2$\stackrel{��ȼ}{��}$12CO2+6H2O |
| A�� | N2�ǻ�ԭ���CO2���������� | |
| B�� | ÿ����22.4LCO2��ת��3 mol���� | |
| C�� | ��Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ4��3 | |
| D�� | NԪ�ؼȱ�������Ҳ����ԭ |