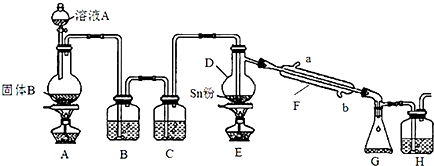
��Ŀ����
19��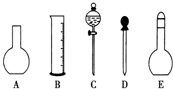 ʵ������Ҫ0.1mol•L-1 NaOH��Һ450mL��0.5mol•L-1������Һ500mL��������������Һ����������ش��������⣺
ʵ������Ҫ0.1mol•L-1 NaOH��Һ450mL��0.5mol•L-1������Һ500mL��������������Һ����������ش��������⣺��1����ͼ��ʾ��������������Һ�϶�����Ҫ����_AC������ţ�������������Һ�����õ��IJ��������Dz��������ձ������������ƣ���
��2�����в����У�����ƿ�����߱��Ĺ�����BCDF������ţ���
A������һ�����ȷŨ�ȵı���Һ B��������Һ
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
E����ȡһ�������Һ��
F�����������ܽ��������
��3�����ݼ�����������ƽ��ȡNaOH������Ϊ2.0g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ�ȴ���0.1mol•L-1������ڡ������ڡ���С�ڡ�����ͬ����
��4�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g•cm-3��Ũ��������Ϊ13.6mL������������һλС������
���� ��1�����ݲ�������ѡȡʵ��������
��2������m=nM=CVM�����������Ƶ�����������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��3����������ƿ�Ľṹ�ص���ʹ��ԭ����
��4���ȼ���Ũ�������ʵ���Ũ�ȣ�����Ũ��Һϡ��ǰ�����ʵ����ʵ����������Ũ����������
��� �⣺��1�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ������ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ����Բ���Ҫ��������AC������Ҫ���������ձ��Ͳ�������
�ʴ�Ϊ��AC�����������ձ���
��2������ƿ�����������桢������Һ���ܽ�������ʵȣ�ֻ��1���̶��߲��ܲ�������ƿ������µ����������Һ�壬�ʴ�Ϊ��BCDF��
��3��m=nM=CVM=0.1mol/L��0.5L��40g/mol=2g��������ƿ�ж���ʱ��������ƿ�̶��ߣ�������Һ�����ƫС������������Һ��Ũ��ƫ�ʴ�Ϊ��2.0�����ڣ�
��4��Ũ��������ʵ���Ũ��Ϊ��c=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L������ҪŨ��������ΪV��0.5mol/L��0.5L=18.4mol/L��V��V=0.0136L=13.6mL���ʴ�Ϊ��13.6��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ��Ƚϻ�����ע���c=$\frac{n}{V}$��������ԭ���������������ƹ��̣�

| A�� | �������������ƽ��Ħ����������ʱ����Ӧ�ﵽ��ƽ�� | |
| B�� | ������������ѹǿ����ʱ����Ӧ�ﵽƽ�� | |
| C�� | �����¶ȣ�ƽ�������ƶ� | |
| D�� | ƽ������X��������Ӧ�ġ�H���� |
���飺��ˮ ������������Һ �����Ȼ�̼ ��ʳ��
���飺A���ᾧ B������ C����ȡ D����Һ E���ܽ�
| ��� | ����� | �Լ���� | ������� |
| ��1�� | ����غ��Ȼ��ƵĹ������� | ||
| ��2�� | �Ȼ��غ͵�Ļ����Һ | ||
| ��3�� | ���ͺ�ˮ�Ļ���� | ||
| ��4�� | �Ҷ������е�198�棩�ͱ��������е�290�棩�Ļ���� | �� |
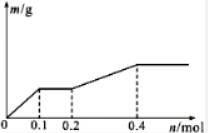 ��Pt�缫��⺬��Ag+��Cu2+��X3+��0.1mol����Һ�����������������ʵ�����m��g�����·��ͨ�����ӵ����ʵ���n��mol���Ĺ�ϵ��ͼ��ʾ��������������ǿ�����ж���ȷ���ǣ�������
��Pt�缫��⺬��Ag+��Cu2+��X3+��0.1mol����Һ�����������������ʵ�����m��g�����·��ͨ�����ӵ����ʵ���n��mol���Ĺ�ϵ��ͼ��ʾ��������������ǿ�����ж���ȷ���ǣ�������| A�� | Ag+��X3+��Cu2+��H+��X2+ | B�� | Ag+��Cu2+��X3+��H+��X2+ | ||
| C�� | Cu2+��X3+��Ag+��X2+��H+ | D�� | Cu2+��Ag+��X3+��H+��X2+ |
| A�� | Na2CO3��Һ��ˮϡ�ͺָ���ԭ�¶ȣ�pH��KW����С | |
| B�� | �����£�pH=2��һԪ���pH=12��һԪǿ��������ϣ�C��OH-��=C��H+�� | |
| C�� | 0.1mol•L-1NaOH��Һ�ֱ��к�pH���������ͬ�Ĵ�������ᣬ������Na0H��Һ�����ǰ��С�ں��� | |
| D�� | 0.1mol•L-1CH3COONa��Һ��0.1mol•L-1CH3COOH��Һ��Ϻ���ҺpH��7��c��CH3COO-����c��Na+����c��CH3COOH����c��H+����c��OH-�� |
| A�� | ��¯�г�����ˮ���ݿ�����ϡ�����ܽ�ȥ�� | |
| B�� | ����FeCl3������Һ�Ʊ�Fe��OH��3�ٽ���Fe3+ˮ�� | |
| C�� | ��ˮ�м���������¶���ʹˮ�����ӻ���С��ˮ�ĵ���ƽ�ⶼ�����ƶ� | |
| D�� | ��Ӧ2A��g��+B��g���T3C��s��+D��g����һ�����������Է����У�˵���÷�Ӧ�ġ�H��0 |
| A�� | H2O2�ĵ���ʽ��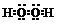 | |
| B�� | �Ҵ��Ľṹʽ��C2H6O | |
| C�� | ��ԭ�ӵĽṹʾ��ͼ�� | |
| D�� | FeSO4 �ĵ��뷽��ʽ��FeSO4=Fe3++SO42�� |
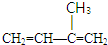 ��
��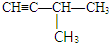 ��
��