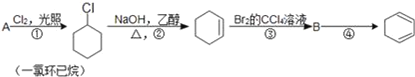
��Ŀ����
����Ŀ���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�顣��ش��������⣺
��.����к͵ζ�������֪ijNaOH�����к���NaCl���ʣ�Ϊ�ⶨ������NaOH��������������������ʵ�飺
�ٳ���1.00 g��Ʒ����ˮ�����250 mL��Һ��
��ȷ��ȡ25.00 mL������Һ����ƿ�У�
�۵μӼ��η�̪��Һ��
����0.10 mol/L�������Һ�ζ����Σ�ÿ����������������¼���£�
�ζ���� | ����Һ���mL | �����������Һ�����/mL | |
�ζ�ǰ���� | �ζ������ | ||
1 | 25.00 | 0.50 | 20.60 |
2 | 25.00 | 6.00 | 26.00 |
3 | 25.00 | 1.10 | 21.00 |
(1)��__________�ζ���(������ʽ��������ʽ��)ʢװ0.10 mol/L�������Һ
(2)������NaOH����������Ϊ__________
(3)����������������ⶨ���ƫ�ߵ���________
A.�ζ�ǰ������ˮ��ϴ��ƿ
B.������ƿʱ������ƿ����Һ����
C.���ڵζ������в�����������Һ������ƿ��
D.��ʽ�ζ��ܵ����յ�ʱ�����Ӷ���
E.��ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ
��.������ԭ�ζ�
(4)ȡ������Һ������ƿ�У���������ϡ���ᣬ��Ũ��Ϊ0.1 mol��L-1�ĸ��������Һ�ζ��������ķ�ӦΪ2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O�ζ�ʱ��KMnO4��ҺӦװ����ʽ�ζ����У��ζ��յ�ʱ������__________
(5)��0.01 mol/L��I2����Һ�ζ�δ֪Ũ�ȵ�Na2S2O3��Һ��ѡ�õ�ָʾ����__________
���𰸡���ʽ 80% CE ���������һ�θ��������Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ ������Һ
��������
(1)��ʽ�ζ�������ʢ�����Ի���ǿ��������Һ����ʽ�ζ�������ʢ�ż�����Һ��
(2)�ȷ������ݵ���Ч�ԣ�������������ƽ�������Ȼ����ݹ�ϵʽNaOH��HCl���NaOH�����ʵ������ټ���NaOH������������
(3)����c(����)=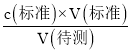 ����������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
����������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
(4)�����������ˮ��Һ����ɫ������������Ϊ��ɫ��
(5)���ݵⵥ����������Һ��Ϊ��ɫ�����жϡ�
(1)0.10 mol/L�������ҺΪ������Һ��Ӧѡ����ʽ�ζ��ܣ�
(2)����������������ֱ�Ϊ��20.10 mL��19.90 mL��21.00 mL������������ƫ��̫��Ӧ����ȥ�������������ƽ�����Ϊ20.00 mL�����ݷ�Ӧ����ʽNaOH+HCl=NaCl+H2O��֪n(NaOH)=n(HCl)=c��V=0.10 mol/L��0.020 L=0.00200 mol������25.00 mL������Һ����m(NaOH)=n��M=0.00200 mol��40 g/mol=0.08g�����250 mL������Һ����m(NaOH)=0.0800 g��![]() =0.8 g����������NaOH������������Ϊ��(NaOH)=
=0.8 g����������NaOH��������������(NaOH)=![]() ��100%=80%��
��100%=80%��
(3)A���ζ�ǰ������ˮ��ϴ��ƿ�������ı�Һ���������Ӱ�죬����c(����)=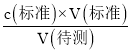 ��������ҺŨ�Ȳ��䣬A���������⣻
��������ҺŨ�Ȳ��䣬A���������⣻
B��������ƿʱ������ƿ����Һ�������������ı�Һ���ƫС�����ݵζ���ʽ��֪������ҺŨ��ƫ�ͣ�B���������⣻
C�����ڵζ������в�����������Һ������ƿ�⣬�������ı�Һ���ƫ���ݵζ���ʽ��֪������ҺŨ��ƫ�ߣ�C�������⣻
D����ʽ�ζ��ܵ����յ�ԣ����Ӷ������������ı�Һ���ƫС�����ݵζ���ʽ��֪������ҺŨ��ƫ�ͣ�D���������⣻
E����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ���������ı�Һ���ƫ���ݵζ���ʽ��֪������ҺŨ��ƫ�ߣ�E�������⣻
�ʺ���ѡ����CE��
(4)�ζ�ʱ��KMnO4��ҺӦװ����ʽ�ζ����У���ƿΪ��ɫ���ᣬ��ʼ�ζ�ʱ������Һ��������ҺΪ��ɫ�����ﵽ�յ�ʱ����ƿ����Һ����ɫ��Ϊdz��ɫ��dz��ɫ���Ұ�����ڲ���ɫ��
(5)��0.01 mol/L��I2����Һ�ζ�δ֪Ũ�ȵ�Na2S2O3��Һ�����ζ���ȫ��I2�������ɸ���I2��������Һ���Ϊ��ɫ�����Կɾݴ�ѡ�õ�����ҺΪָʾ����

����Ŀ������ʽ�ζ���ȷ��ȡ25.00mLijδ֪Ũ�ȵ�������Һ��һ�ྻ����ƿ�У�Ȼ����0.2000mol��L-1������������Һ(ָʾ��Ϊ��̪)�ζ����ζ����������ʾ��
NaOH��Һ��ʼ���� | NaOH�յ���� | |
��һ�� | 0.10mL | 18.60mL |
�ڶ��� | 0.30mL | 19.00mL |
��1��ȷ����0.2000mol��L��1������������Һ250mL����Ҫ����Ҫ��������Ͳ���ձ����������⣬�������õ��IJ���������___��
��2�������������ݿ��Լ������������ʵ���Ũ��Ϊ___ mol��L-1��(����4λ��Ч����)
��3����0.2000mol��L-1������������Һ�ζ�����������Һ���ζ�ʱ���ֿ��Ƽ�ʽ�ζ��ܵIJ��������ֲ�ͣҡ����ƿ���۾�ע��___��ֱ���ζ��յ㡣
��4���ﵽ�ζ��յ�ı�־��___��
��5�����²�����ɲⶨ���ƫ�ߵ�ԭ�������___ (����ĸ����)��
A.δ�ñ�Һ��ϴ��ʽ�ζ���
B.�ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
C.ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
D.�ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
����Ŀ���±���¼��t��ʱ��4����ͬ������ͭ��Һ�м�����ˮ����ͭ�������Լ����������� ͭ����(CuSO4��5H2O)������(�¶ȱ��ֲ���)��ʵ����駣�
����ͭ��Һ | �� | �� | �� | �� |
�������ˮ����ͭ(g) | 3.00 | 5.50 | 8.50 | 10.00 |
����������ͭ����(g) | 1.00 | 5.50 | 10.90 | 13.60 |
������6.20g��ˮ����ͭʱ����������ͭ���������(g)Ϊ
A.7.70B.6.76C.5.85D.9.00