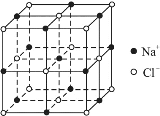
��Ŀ����
����Ŀ���������ߣ��������Ͽ�֪�����ʾ���ݣ�
���� | �Ҵ� | ���� | �������� | Ũ���� |
�е�/�� | 78.5 | 117.9 | 77.5 | 338.0 |
[ʵ�鲽��]
ijѧ����ʵ������ȡ������������Ҫ�������£�
����30 mL�Ĵ��Թ�A�а������1��4��4����Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ��ʾ���Ӻ�װ��(װ������������)����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10 min��
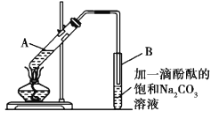
�۴��Թ�B�ռ���һ�����IJ����ֹͣ���ȣ���ȥ�Թ�B��������Ȼ���ô��ֲ㣻
�ܷ�������������㡢ϴ�ӡ����
�������ĿҪ��ش��������⣺
(1)д����ȡ���������Ļ�ѧ����ʽ��___________________________��
(2)B�Թ��ñ���̼��������������ռ�����������_____________________��
(3)���������������Ϊ�˸�������������ѡ�õĸ����Ϊ__________(����ĸ)��
A.P2O5 B.��ˮNa2SO4 C.��ʯ�� D.NaOH����
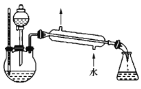
(4)ij��ѧ����С���������ͼ��ʾ����ȡ����������װ��(ͼ�е�����̨�����С�����װ������ȥ)������ͼװ����ȣ���װ�õ���Ҫ�ŵ���__________________��
���𰸡�CH3COOH+C2H5OH![]() CH3COOC2H5+H2O �к����ᣬ�����Ҵ�������Ϊ���������ڱ���̼������Һ�е��ܽ��С�������ڷֲ����� B ���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����IJ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����������������װ�ã��������ռ�������������
CH3COOC2H5+H2O �к����ᣬ�����Ҵ�������Ϊ���������ڱ���̼������Һ�е��ܽ��С�������ڷֲ����� B ���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����IJ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����������������װ�ã��������ռ�������������
��������
���������������Ʊ�ԭ����������ʵ����ʲ����װ��ͼ�������
��1���������Ҵ���Ӧ��������������ˮ����ѧ��Ӧ����ʽ��CH3COOH+C2H5OH![]() CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+C2H5OH
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+C2H5OH![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��2������̼���ƿ����кͻӷ������ᣬ�����Ҵ�������Ϊ���������ڱ���̼������Һ�е��ܽ��С�������ڷֲ��������ʴ�Ϊ���к����ᣬ�����Ҵ�������Ϊ���������ڱ���̼������Һ�е��ܽ��С�������ڷֲ�������
��3�����������ֲ�Ʒ���ᴿ��������Ϊ������ֲ�Ʒ�м���̼���Ʒ�ĩ(Ŀ���dz�ȥ�ֲ�Ʒ�е�����)���������м��뱥��ʳ��ˮ�뱥���Ȼ�����Һ�������á���Һ(Ŀ���dz�ȥ�ֲ�Ʒ�е��Ҵ�)���������м�����ˮ������(Ŀ���dz�ȥ�ֲ�Ʒ�е�ˮ)��������������������Һ�������һ���������ƿ�ڣ���������ȥ�ͷе���֣��ռ��¶���76��78��֮�����ּ��ô����������������Ի���Ը��������ʹ��������ˮ�⣬���Ը�������������ѡ�õĸ����Ϊ��ˮNa2SO4��ѡB���ʴ�Ϊ��B��
��4���Ա�����ʵ��װ��ͼ��������������Ʊ������еĸ����������ƣ����Կ������ߵ�����ͻ�����ŵ㣺���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����IJ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�������������ˮ����װ�ã��������ռ����������������ʴ�Ϊ�����������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����IJ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�����������������װ�ã��������ռ���������������