��Ŀ����
�±�Ϊ���ڱ���һ���֣����еı�Ŵ�����Ӧλ�õ�Ԫ�ء�
|
�� |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�� |
|
|
|
|
|
|
|
|
|
|
|
�� |
�� |
�� |
�� |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�� |
|
|
|
�� |
|
|
|
|
|
|
|
�� |
|
|
|
|
�� |
|
|
|
|
|
|
|
��ش��������⣺
��1��д���ϱ���Ԫ�آ�ԭ�ӵĺ�������Ų�ʽ ��
��2��Ԫ�آܡ��ݵĵ�һ�����ܴ�С˳���ǣ� �� ������Ԫ�ط��ű�ʾ����ͬ��Ԫ�آޡ���縺�Դ�С˳���ǣ� ��
��3��Ԫ�آۡ����γɵĻ���������ԭ���ӻ�������ͣ� �����ӵĿռ乹��
��4����֪ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ���Ԫ�آ���Ԫ�آߵ���
�����������Ƶ����ʡ�д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
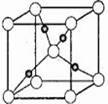
��5����ͼ�Ǣ��ij��������ľ����ṹʾ��ͼ�����Ǻ���������������ȷ����������Ļ�ѧʽΪ ��
��6��������ͭ��Һ�м��������ˮ��������[Cu(NH3)4]2�������ӡ�
��֪NF3��NH3�Ľṹ���ƣ���NF3 ������Cu2���γ������ӣ�����Ҫԭ���ǣ�
(��16�� ÿ�ո�2��)
��1��1S22S22P63S23P63d 54s1��2��N���� ��F��Cl��3��sp3�ӻ�������������
��4��Be(OH)2+2NaOH===2H2O+Na2BeO2 ��5��Cu2O
��6��NF3�����з�ԭ�ӵ縺��ǿ��������������ǿ��ʹ�õ�ԭ���ϵŶԵ���������Cu2���γ���λ����
����������1�����Ǹ�Ԫ�أ����ݹ���ԭ����֪����ԭ�Ӻ�����ӵ��Ų�ʽΪ1S22S22P63S23P63d 54s1��
��2���ܢݷֱ���N��O���ǽ�����Խǿ����һ������Խ�����ڵ�ԭ�ӵ�2p��������ǰ����״̬���ȶ���ǿ�����Ե�Ԫ�صĵ�һ�����ܴ�����Ԫ�صġ��ޢ�ֱ���F��Cl���ǽ�����Խǿ���縺��Խ���ķǽ�����ǿ��Cl�ģ����Է��ĵ縺�Դ����ȵġ�
��3���ۢ��γɵ�CCl4�����У�����ԭ�ӵŶԵ����ǣ�4��1��4����2��0�ԣ����������������νṹ��̼ԭ����sp3�ӻ���
��4������B����������������������������������Ը����������ƿ�֪����ӦʽΪ
Be(OH)2+2NaOH===2H2O+Na2BeO2��
��5������ͭ�����ݾ����Խṹ��֪�������к�����ԭ����8��1/8+1=2������ͭԭ����4�������Ի�ѧʽΪCu2O��
��6�����ڷ�ԭ������ǿ�Էǽ���Ԫ�أ����NF3�����з�ԭ�ӵ縺��ǿ��������������ǿ��ʹ�õ�ԭ���ϵŶԵ���������Cu2���γ���λ����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���2011?������ģ��X��Y��Z��W��Ϊ����������Ԫ�أ�X��W��������֮��Ϊ23���±�Ϊ���ڱ���һ���֣�����˵����ȷ���ǣ�������
|
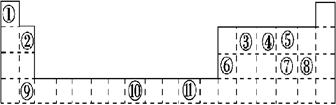
��13�֣��±�Ϊ���ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
|
a |
|
|
|
|
|||||||||||||
|
|
|
|
|
b |
c |
d |
|
|
|||||||||
|
|
|
|
|
|
e |
f |
|
|
|||||||||
|
|
|
|
|
|
g |
|
h |
|
|
|
|
|
|
|
|
|
|
�û�ѧ����ش��������⣺
��1��д��Ԫ��g�Ļ�̬ԭ�Ӻ�������Ų�ʽ___________________________��
h2+��δ�ɶԵ�����Ϊ ��
��2����b2a2�����У�Ԫ��bΪ �ӻ����÷����� ���ӣ�����ԡ��Ǽ��ԡ������÷����ЦҼ��ͦм�����Ŀ��Ϊ ��
��3�� bd2��bf2�Ƚϣ��е�ϸߵ���_______�������ʽ����ԭ���� ��
��4�����й���Ԫ����Ԫ�����ڱ��е�λ���Լ�Ԫ��ԭ�ӵ���Χ�����Ų��ص���й�����ȷ�� ��
A��hλ��Ԫ�����ڱ��е������ڵ�VIII�壬����d��Ԫ��
B��e�Ļ�̬ԭ���У�3p�ܼ�Ϊ�����������p��Ԫ��
C�����������Ų�ʽΪ4s2��һ������IIA��
D�����������Ų�ʽΪns2np1����Ԫ�ؿ����Ǣ�A����B��
��5����ѧ�о�������Ԫ��b��Ԫ��c�����γ�һ�ֳ�Ӳ����ĥ�����µ��������ǽ������ϣ��仯ѧʽΪ �����۵�Ƚ��ʯ ����ߡ��͡�����


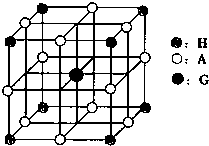
 ��۵����Ų�ʽ__________�����������е����Ų�4s����ϵ�������֮��ͬ�Ļ��� Ԫ��(��Ԫ�ط���)��
��۵����Ų�ʽ__________�����������е����Ų�4s����ϵ�������֮��ͬ�Ļ��� Ԫ��(��Ԫ�ط���)��