��Ŀ����
A��B��C��D��E�����л�����Ƿ�����C��H��O����Ԫ�ص������ȶ���6��1��8����ͨ��״���£�A��һ���д̼�����ζ�����壬������������ܶ�Ϊ15����ˮ��Һ�ܷ���������Ӧ��B����Է���������A��6����C��B��ͬ���칹�壬�����ʶ��Ǿ�����ζ����ɫ���壬��B�������ƾ���ҵ�Ļ�ԭ����D��E�����ʵ������ܶȶ���2.68 g��L��1(��״����)������Ҳ��Ϊͬ���칹�塣��D��ˮ��Һ��ʹʯ����Һ��죬��E�Dz�����ˮ����״Һ�壬����ˮ����ζ����д��A��B��C��D��E�����ƺͽṹ��ʽ��
A________��________��
B________��________��
C________��________��
D________��________��
E________��________��
A________��________��
B________��________��
C________��________��
D________��________��
E________��________��
��ȩ��HCHO
�����ǡ�CH2OH(CHOH)4CHO
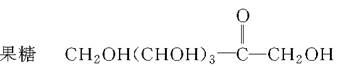
���ᡡCH3COOH
���������HCOOCH3
�����ǡ�CH2OH(CHOH)4CHO
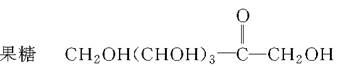
���ᡡCH3COOH
���������HCOOCH3
�������ʷ�����C��H��O��ԭ�Ӹ�����N(C)��N(H)��N(O)��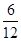 ��
�� ��
�� ��1��2��1�����ԣ����ʽΪCH2O������A��ʽ��Ϊ30������һ���д̼�����ζ�����壬��AΪ��ȩ��B��C��ʽ����Ϊ30��6��180������ʽΪC6H12O6������B�����ƾ���ҵ�Ļ�ԭ������BΪ�����ǣ�CΪ���ǡ�D��E��Ħ������Ϊ22.4 L��mol��1��2.68 g��L��1��60 g��mol��1������ʽΪC2H4O2��D��ˮ��Һ�����ԣ�EΪ������ˮ����״Һ�壬��DΪ���ᣬEΪ���������
��1��2��1�����ԣ����ʽΪCH2O������A��ʽ��Ϊ30������һ���д̼�����ζ�����壬��AΪ��ȩ��B��C��ʽ����Ϊ30��6��180������ʽΪC6H12O6������B�����ƾ���ҵ�Ļ�ԭ������BΪ�����ǣ�CΪ���ǡ�D��E��Ħ������Ϊ22.4 L��mol��1��2.68 g��L��1��60 g��mol��1������ʽΪC2H4O2��D��ˮ��Һ�����ԣ�EΪ������ˮ����״Һ�壬��DΪ���ᣬEΪ���������
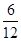 ��
�� ��
�� ��1��2��1�����ԣ����ʽΪCH2O������A��ʽ��Ϊ30������һ���д̼�����ζ�����壬��AΪ��ȩ��B��C��ʽ����Ϊ30��6��180������ʽΪC6H12O6������B�����ƾ���ҵ�Ļ�ԭ������BΪ�����ǣ�CΪ���ǡ�D��E��Ħ������Ϊ22.4 L��mol��1��2.68 g��L��1��60 g��mol��1������ʽΪC2H4O2��D��ˮ��Һ�����ԣ�EΪ������ˮ����״Һ�壬��DΪ���ᣬEΪ���������
��1��2��1�����ԣ����ʽΪCH2O������A��ʽ��Ϊ30������һ���д̼�����ζ�����壬��AΪ��ȩ��B��C��ʽ����Ϊ30��6��180������ʽΪC6H12O6������B�����ƾ���ҵ�Ļ�ԭ������BΪ�����ǣ�CΪ���ǡ�D��E��Ħ������Ϊ22.4 L��mol��1��2.68 g��L��1��60 g��mol��1������ʽΪC2H4O2��D��ˮ��Һ�����ԣ�EΪ������ˮ����״Һ�壬��DΪ���ᣬEΪ���������
��ϰ��ϵ�д�
�����Ŀ
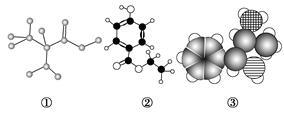
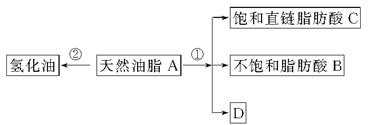
 ���л��������ϡ����·���ˮ�ⷴӦ����ˮ�����ɲ���ֻ��һ��
���л��������ϡ����·���ˮ�ⷴӦ����ˮ�����ɲ���ֻ��һ��